Our mission
The overall mission of the Roswell Center for Quantitative Imaging (ROCQI) is to accelerate the development of advanced imaging solutions, including artificial intelligence (AI), machine learning techniques, and radiomics, for research applications.
We aim to serve as the scientific and technological hub that fosters both intra- and inter-institutional collaborations in the fields of quantitative imaging and radiomics.
What we do
The Center for Quantitative Imaging is the academic home for an integrated team of basic and translational scientists, information technology specialists and clinicians who work closely to facilitate imaging studies via machine learning methods.
Our research programs focus on:
- Advanced Imaging Techniques: Developing and refining innovative imaging technologies to enhance diagnostic accuracy, including AI-driven analysis, high-resolution imaging, and real-time imaging techniques.
- Imaging Biomarkers: Identifying and validating new biomarkers that can be detected through imaging. This includes research into how imaging can be used to predict disease progression, treatment response, and patient prognosis.
- Personalized Medicine: Utilizing imaging data to tailor cancer treatment plans to individual patients, improving outcomes and minimizing side effects. This involves correlating imaging findings with genetic, molecular, and clinical data.
- Machine Learning and Data Analytics: Applying machine learning algorithms to large imaging datasets to discover patterns and insights that can improve diagnosis, treatment, and understanding of cancer.
- Collaborative Research Initiatives: Engaging in multidisciplinary collaborations with academic institutions, healthcare providers, and industry partners to drive innovation and translate research findings into clinical practice.
Ongoing research projects are funded by the National Cancer Institute (NCI) and the Roswell Park Alliance Foundation.
Research highlights
Modern cross-sectional imaging shows that multiple myeloma infiltrates the bone marrow with different patterns: While some patients have a diffuse infiltration, others show focal lesions or a mixed pattern of both. The focal lesions are particularly relevant since they can lead to osteolytic lesions with increased fracture risk or extramedullary disease which has been shown to be of adverse prognostic significance.
The use of immunotherapies, especially treatment with CAR-T cells has shown to occasionally cause an increase in the size of focal lesions while other markers of the disease show treatment response which suggests a phenomenon known as pseudo-progression in solid tumors.
In an international collaboration with centers around the world we aim to classify the phenomenon of pseudo-progression in patients with multiple myeloma and are utilizing an online platform where investigators can upload both clinical as well as imaging data so that radiologists and biostatisticians can access this data and perform their evaluations.
We hope that this platform will also be useful for other projects necessitating collaborations between different sites.
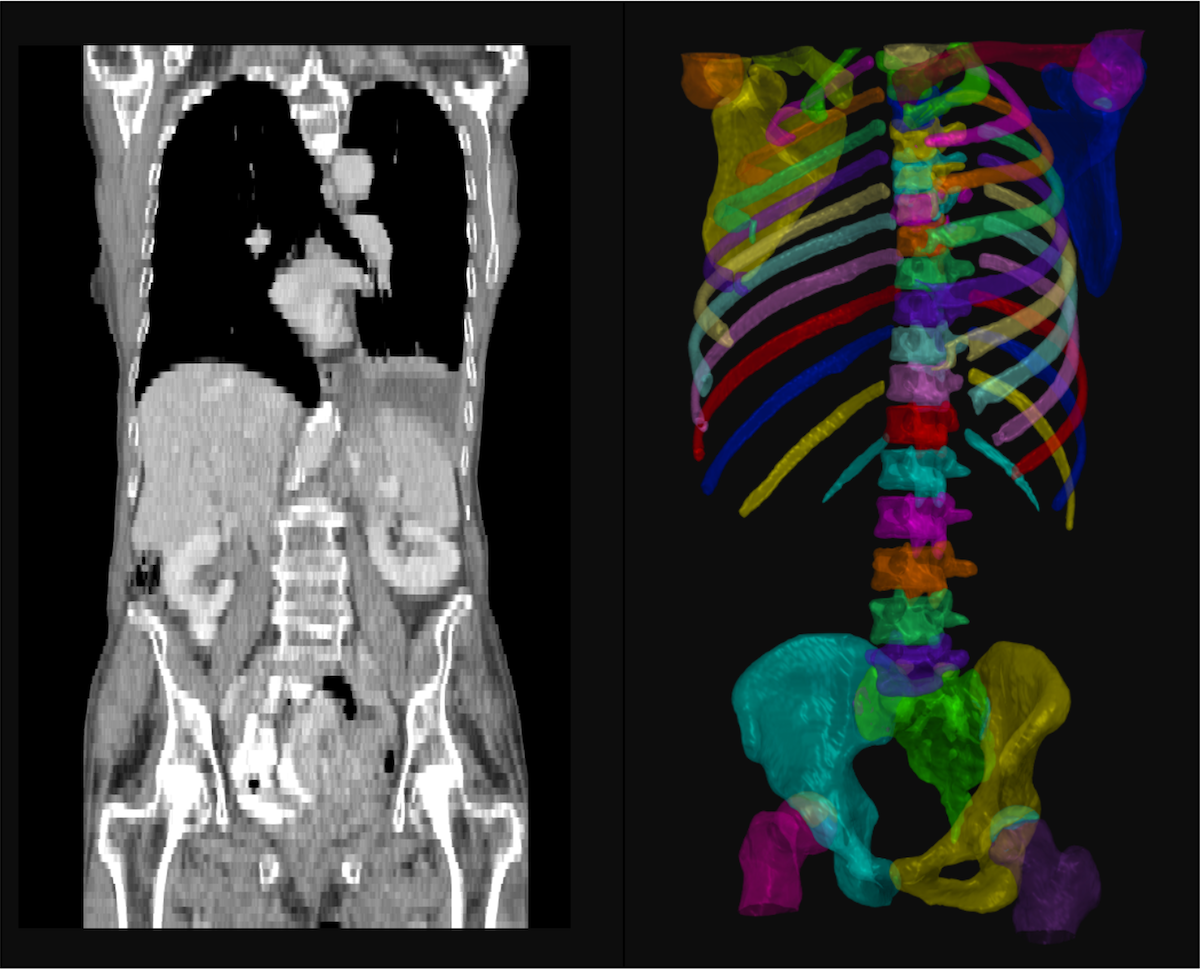
Osteolytic lesions and bone fractures are frequent symptoms of multiple myeloma and can lead to significant morbidity and mortality. Modern imaging techniques allow for longitudinal assessment of the skeletal system to monitor these changes.
We aim to analyze MRI, PET and CT images from patients with smoldering multiple myeloma (an asymptomatic precursor of multiple myeloma) and multiple myeloma to retrospectively identify imaging markers that allow to predict a fracture in subsequent imaging. Because of the amount of imaging data that will have to be reviewed we are developing computer-based tools to perform these analyses in a reasonable period of time.
This will allow to intervene earlier and prevent fractures and further bone damage and help predict fracture risk for example of patients who are planning to go back to work or to participate in physical exercise and other leisure activities.
Read more:
- Klein A, Warszawski J, Hillengass J, Maier-Hein KH. Automatic bone segmentation in whole-body CT images. Int J Comput Assist Radiol Surg. 2019 Jan;14(1):21-29. doi: 10.1007/s11548-018-1883-7. Epub 2018 Nov 13. PMID: 30426400
- Hildenbrand N, Klein A, Maier-Hein K, Wennmann M, Delorme S, Goldschmidt H, Hillengass J. Identification of focal lesion characteristics in MRI which indicate presence of corresponding osteolytic lesion in CT in patients with multiple myeloma. Bone. 2023 Oct;175:116857. doi: 10.1016/j.bone.2023.116857. Epub 2023 Jul 22. PMID: 37487861
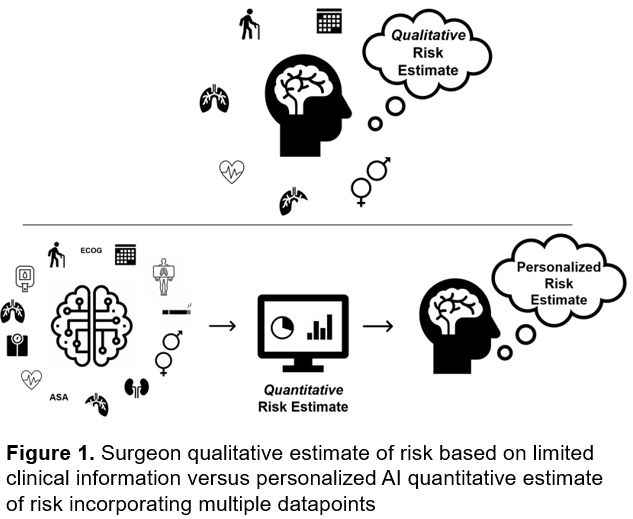
Our proposed research aims to develop an AI-driven predictive surgical risk stratification tool for lung cancer patients. Given the high risks associated with lung cancer surgery, precise preoperative risk assessments are critical. Current methods rely on subjective judgments and outdated tests, leading to inaccuracies. This new model will integrate AI to analyze preoperative CT imaging and clinical data, providing a quantitative and personalized risk assessment to better identify candidates for surgery or alternative treatments.
The innovation lies in combining radiomic data with clinical data through AI, enhancing the accuracy of risk predictions. This model could transform thoracic surgery workflows, expanding surgical eligibility and improving patient outcomes.
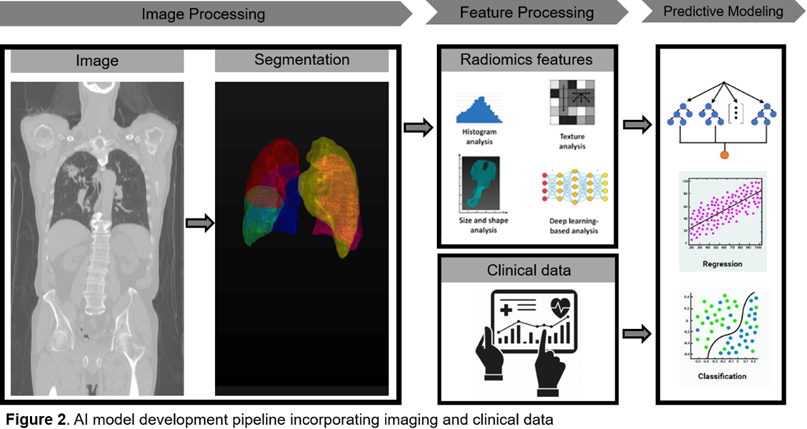
The approach includes data acquisition, preprocessing, feature selection, model engineering, and rigorous validation. The model will be trained on a comprehensive dataset of lung cancer surgeries, using advanced machine learning algorithms to identify the most predictive features. Additionally, the study will explore the impact of CT imaging parameters on predictive accuracy and compare the AI model's performance with traditional methods. The ultimate goal is to improve postoperative outcomes for lung cancer patients and set the stage for similar predictive models in other medical fields.
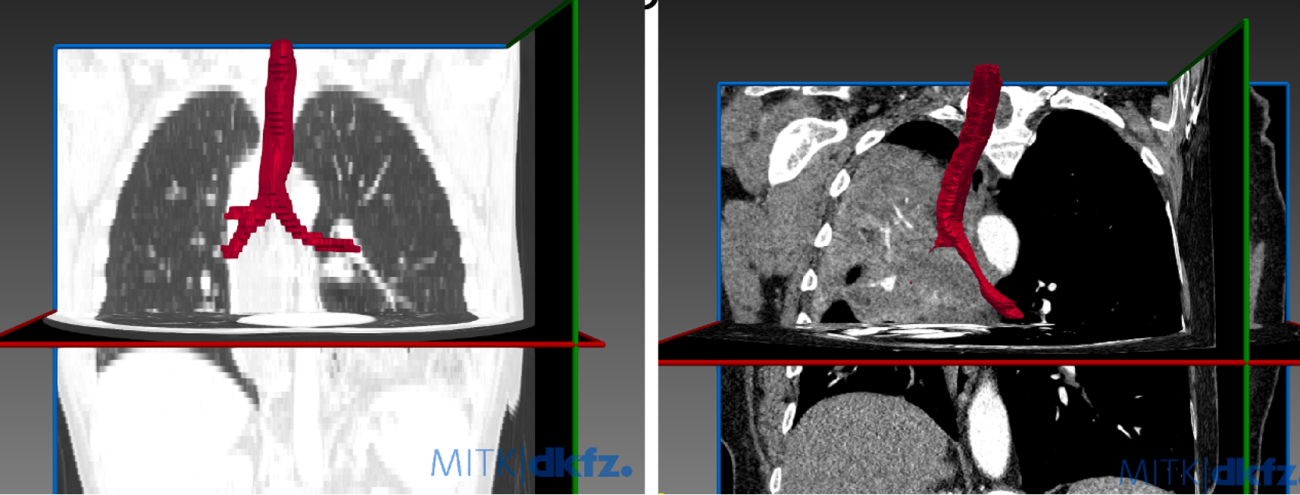
Malignant Central Airway Obstruction is a common complication of lung cancer, with 17% having at least mild obstruction at time of lung cancer diagnosis, and 8.2% of additional patients developing airway obstruction over the subsequent 5 years. We have demonstrated that airway obstruction can be identified on dedicated review of CT scans in 99% of cases, as compared to the gold standard of bronchoscopy. Despite this, radiologists do not identify airway obstruction in their reads in up to 30% of cases. We propose to develop a computer algorithm to automatically segment the central airways and accurately characterize the presence of central airway obstruction.
Strategy:
- We have selected a teaching cohort of CT scans both with an without central airway obstruction.
- We used MITK open source software to segment the airways.
- We compared airway segmentation between researchers and then against the algorithm.
- Next steps: We will perform training cohorts of non-segmented airways to determine accuracy in characterizing the airways and identifying airway obstruction.
Further Reading:
- Ivanick NM, Kunadharaju R, Bhura S, Mengiste H, Saeed M, Saradna A, et al. Epidemiology and Survival of Malignant Central Airway Obstruction in Lung Cancer Identified on Cross-Sectional Imaging. Journal of bronchology & interventional pulmonology. 2024;31(3).
- Kalvapudi S, Zubair HM, Kunadharaju R, Bhura S, Mengiste H, Saeed M, et al. Correlation of Bronchoscopy and CT in Characterizing Malignant Central Airway Obstruction. Cancers. 2024;16(7).
- Harris K, Alraiyes AH, Attwood K, Modi K, Dhillon SS. Reporting of central airway obstruction on radiology reports and impact on bronchoscopic airway interventions and patient outcomes. Therapeutic advances in respiratory disease. 2016;10(2):105-12.
- Daneshvar C, Falconer WE, Ahmed M, Sibly A, Hindle M, Nicholson TW, et al. Prevalence and outcome of central airway obstruction in patients with lung cancer. BMJ open respiratory research. 2019;6(1):e000429.
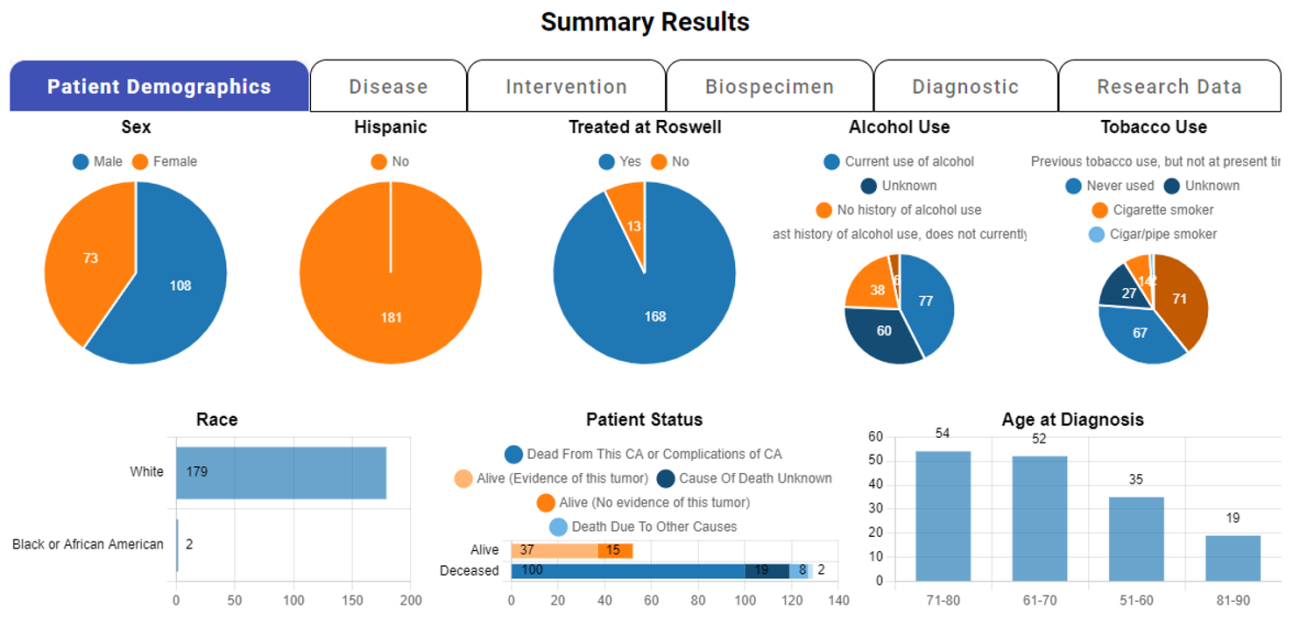
Cohort Discovery
nSight™ is a powerful data discovery platform offered complementary to the entire Roswell team who possess the requisite CITI training certifications. The core mission of the nSight initiative is to enable Roswell researchers with real-time access to vital clinical, research and regulatory data, thereby facilitating their research endeavors. With its diverse range of capabilities, the platform serves various purposes, including research feasibility assessment, grant proposal preparation, poster creation and clinical trial population design, among others.
Within this cutting-edge platform, users are empowered to conduct targeted searches for desired cases, utilizing a wide range of factors such as disease type, treatment modality and biospecimen characteristics.
Radiomics research using artificial intelligence and machine learning
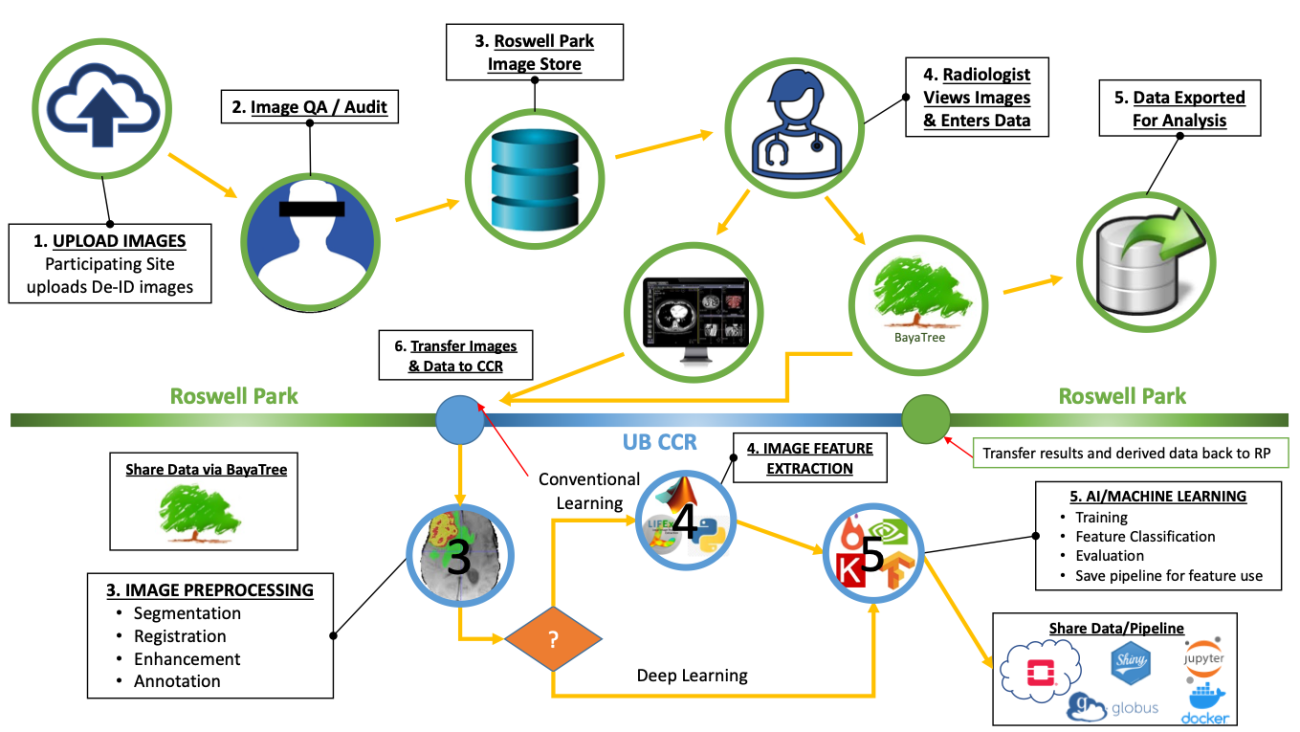
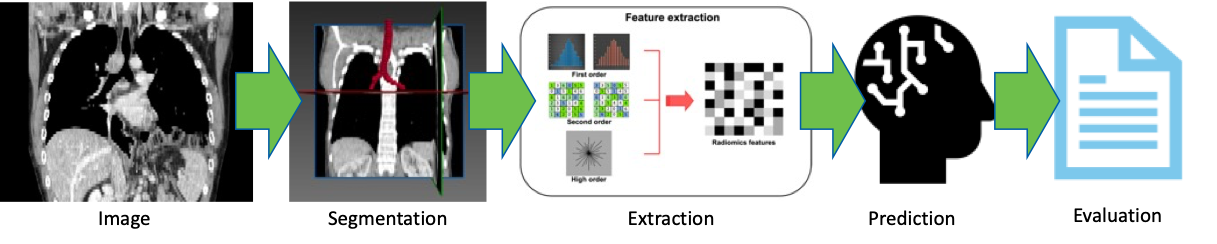
ROCQI is dedicated to harnessing the power of artificial intelligence (AI) and machine learning (ML) techniques to advance research in digital image analysis at Roswell Park. We believe that collaboration across multiple disciplines is essential for successful digital image research. The center focuses on providing comprehensive support to researchers, enabling them to utilize AI and ML algorithms in the analysis of various types of digital images. These images include radiological images, pathology slides and histological images, which play a crucial role in diagnosis, prognosis and treatment planning for cancer patients.
The digital image research pipelines at Roswell Park typically encompass several key steps. ROCQI facilitates image acquisition by collecting digital images from diverse sources. Next, we engage in annotation and segmentation, a process where specific regions of interest within the images are identified and marked, thus creating labeled data for training ML algorithms. Feature extraction is the subsequent step, involving the application of sophisticated algorithms to extract relevant characteristics from the images. These extracted features then serve as inputs for ML models. Finally, we carry out evaluation, which entails assessing the performance and accuracy of the ML models using appropriate metrics. This evaluation process ensures that the ML models are effective and contribute to the advancement of research in digital image analysis at Roswell Park.
The center collaborates closely with researchers to ensure the seamless execution of these steps, with a focus on efficiency and effectiveness. Our aim is to drive innovative research outcomes and facilitate breakthroughs in the analysis of digital images. By leveraging AI and ML techniques, we strive to enhance the speed, accuracy, and precision of diagnoses, as well as the development of personalized treatment plans for cancer patients.
Collaborate with ROCQI
Interested in developing an AI project with us? We look forward to hearing from you!
In the news
Read about the nSight portal, which allows investigators to visualize and integrate data for their research.
Interested in becoming a part of ROCQI?
Looking to gain experience in this emerging field? Become a trainee at the Roswell Center for Quantitative Imaging!
Contact us
Anna Lelonek
Phone: 716-845-1300, x1730
Email: Anna.Lelonek@RoswellPark.org