BMPK is a Certified Service Provider for Olink and for Biocrates.
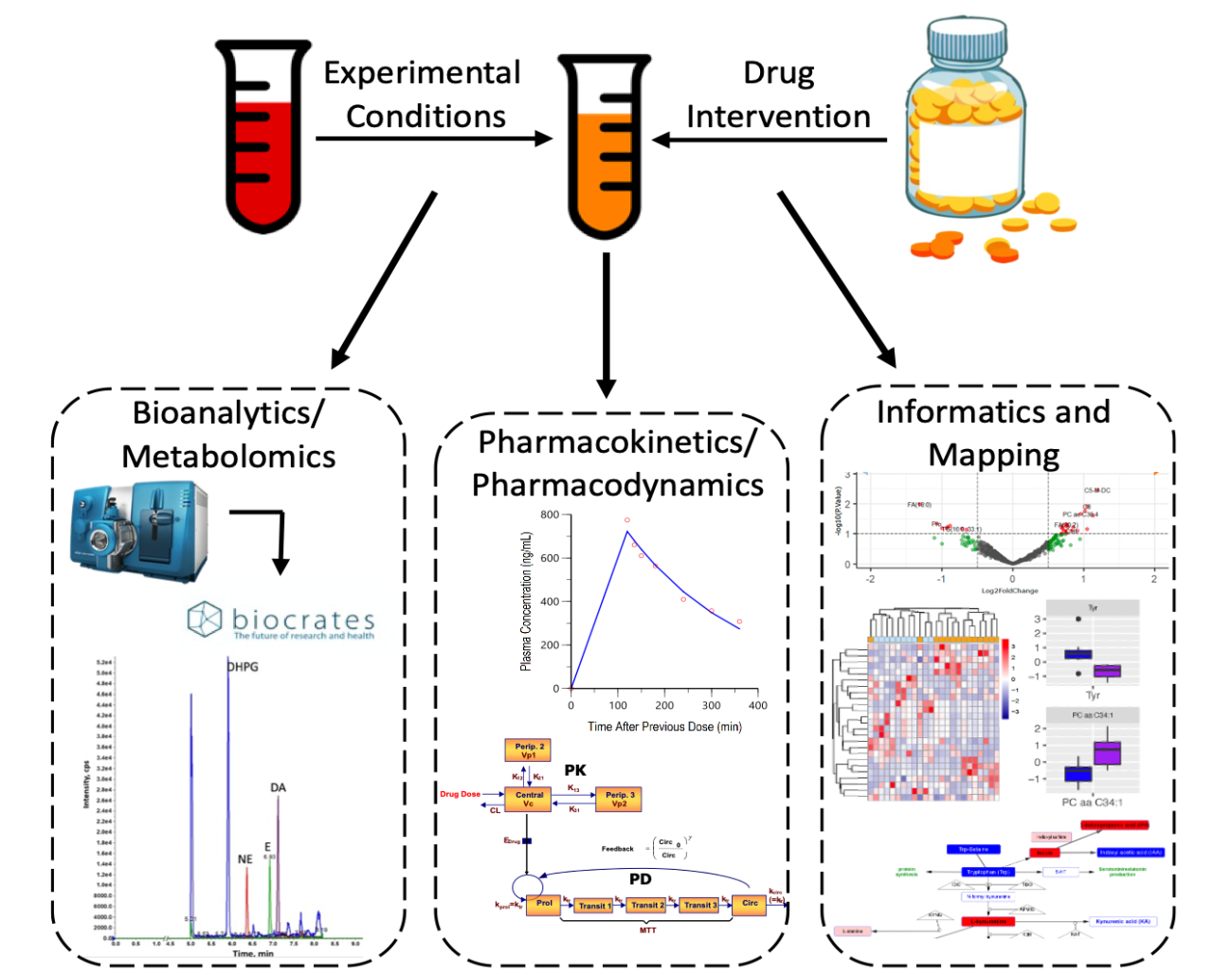
What we do
The Bioanalytics, Metabolomics & Pharmacokinetics Shared Resource (BMPK) provides highly sensitive measurements for an extensive array of chemotherapeutic agents and metabolites to support basic research and preclinical/clinical drug development at Roswell Park Comprehensive Cancer Center, in addition to other cancer centers, academic institutions, and industry.
Investigators are encouraged to consult with BMPK as early as possible during the planning phases of their project or grant development. By starting discussions early, BMPK can advise on proper sample collection and handling and provide dosing recommendations for optimal therapeutic outcomes.
Example categories of drug/metabolite and other small molecule assays using LC-MS/MS for detection:
- Hormones: Testosterone, dihydrotestosterone, androstenedione, dehydroepiandrosterone (DHEA), and androsterone, and estrone and estradiol
- Antimetabolites: Gemcitabine and dFdU, capecitabine, and 5-fluorouracil
- Taxanes: Docetaxel and paclitaxel
- Topoisomerase Agents: Irinotecan (CPT-11), SN-38, SN-38G
- Targeted Agents: Tivozanib, enzalutamide and N-desmethylenzalutamide, sorafenib and sorafenib-N-oxide, carfilzomib, dabrafenib, abemaciclib and abemaciclib M2
Analyte(s) | Lower Limit of Quantitation | Calibration Range | Methodology | Status | Matrices |
|---|---|---|---|---|---|
Androgens (5) |
|
| LC-MS/MS | Validated | Mouse Serum |
Variable | Variable | LC-MS/MS |
| Variable | |
BRAF Inhibitors: |
|
| LC-MS/MS | Validated | Human Plasma |
Calcitriol | 10.0 ng/mL | 10.0 - 1,000 ng/mL | LC-MS/MS | Validated | Drug Formulation |
0.400 ng/mL | 0.400 - 1,000 ng/mL | LC-MS/MS | Validated | Human Heparinized Plasma | |
Catecholamines: |
|
| LC-MS/MS | Validated | Human Plasma |
CDK4/6 Inhibitors: | 5 ng/mL | 5 - 2,000 ng/mL | LC-MS/MS | Validated | Human Plasma |
1.00 ng/mL | 1.00 - 500 ng/mL | LC-MS/MS | Validated | Human Plasma | |
Cytarabine | 1.00 ng/mL | 1.00 - 1,000 ng/mL | LC-MS/MS | Qualified | Human Plasma |
0.200 ng/mL | 0.200 - 400 ng/mL | LC-MS/MS | Validated | Human Plasma | |
Docetaxel | 5.00 ng/mL | 5.00 - 1,000 ng/mL | LC-MS/MS | Qualified | Mouse Plasma |
Dovitinib | 2.00 ng/mL | 2.00 - 1,000 ng/mL | LC-MS/MS | Qualified | Human Heparinized Plasma |
Doxorubicin and Doxorubicinol | 1.0 ng/mL | 1.00-500 ng/mL | LC-MS/MS | Validated | Human Plasma |
Eicosanoids (7) | 1.00 ng/mL | 1.00 - 400 ng/mL | LC-MS/MS | Qualified | Mouse Xenografts |
Enterodiol | 0.500 ng/mL | 0.500 - 150 ng/mL | LC-MS/MS | Validated | Human Serum |
Entinostat | 0.500 ng/mL | 0.500 - 250 ng/mL | LC-MS/MS | Qualified | Human Plasma |
10.0 ng/mL | 10.0 - 20,000 ng/mL | LC-MS/MS | Validated | Human EDTA Plasma | |
Enzalutamide | 10.0 ng/mL | 10.0 - 20,000 ng/mL | LC-MS/MS | Qualified | Woodchuck EDTA Plasma |
Epacadostat | 5.00 ng/mL | 5.00 - 1,000 ng/mL | LC-MS/MS | Validated | Human EDTA Plasma |
2.00 pg/mL | 2.00 - 250 pg/mL | LC-MS/MS | Validated | Human Heparinized Plasma | |
1.00 ng/mL | 1.00 - 2,000 ng/mL | LC-MS/MS | Validated | Human Plasma | |
Gemcitabine | 1.00 ng/mL | 1.00 - 1,000 ng/mL | LC-MS/MS | Qualified | Human Plasma |
Irinotecan (CPT-11), SN-38, and SN-38G | 200 pg/mL | 0.2-200 ng/mL | LC-MS/MS | Qualified | Human Plasma and Tissues |
Metformin | 12.5 ng/mL | 12.5-7500 ng/mL | LC-MS/MS | Qualified | Human and Mouse Plasma |
Nicotine | 1.00 ng/mL | 1.00 - 100 ng/mL | LC-MS/MS | Qualified | Human Urine |
0.500 ng/mL | 0.500 - 250 ng/mL | LC-MS/MS | Validated | Human EDTA Plasma | |
2.00 ng/mL | 2.00 - 1,000 ng/mL | LC-MS/MS | Validated | Human Plasma | |
Pemetrexed | 5.00 ng/mL | 5.00 - 2,000 ng/mL | LC-MS/MS | Qualified | Human Plasma |
1.25 ng/mL | 1.25 - 100 ng/mL | LC-MS/MS | Validated | Human Plasma | |
Sorafenib | 50.0 ng/mL | 50.0 - 10,000 ng/mL | LC-MS/MS | Validated | Human Plasma |
6-Sulfatoxymelatonin |
|
| LC-MS/MS | Validated | Human Urine |
0.0200 ng/mL | 0.020 - 25.0 ng/mL | LC-MS/MS | Validated | Human Plasma | |
(Z)-Tamoxifen | 1.00 ng/mL | 1.00 - 400 ng/mL | LC-MS/MS | Validated | Human Plasma |
TAS102: |
|
|
|
|
|
Various | Varous | LC-MS | Qualified | Various | |
Thiopurine (azathiopurine/6-mercaptopurine) Metabolites: TGN and MMP | 6-TG: 30 ng/mL | 6-TG: 0.03-1.25 ug/mL | LC-MS/MS | Validated | Human Whole Blood |
0.500 ng/mL | 0.500 - 150 ng/mL | LC-MS/MS | Validated | Human EDTA Plasma | |
40.0 ng/mL | 40.0 - 20,000 ng/mL | LC-MS/MS | Validated | Human Plasma | |
Variable | Variable | LC-MS/MS | Validated | Cell Pellet and Media | |
Verteporfin | 0.125 ng/mL | 0.125-50 ng/mL | LC-MS/MS | Validated | Human Plasma |
- Collins CP, Khuat LT, Sckisel GD, Vick LV, Minnar CM, Dunai C, Le CT, Curti BD, Crittenden M, Merleev A, Sheng M, Chao NJ, Maverakis E, Rosario SR, Monjazeb AM, Blazar BR, Longo DL, Canter RJ, Murphy WJ. Systemic immunostimulation induces glucocorticoid-mediated thymic involution succeeded by rebound hyperplasia which is impaired in aged recipients. Front Immunol. 2024 Sep 9;15:1429912. doi: 10.3389/fimmu.2024.1429912. PMID: 39315105; PMCID: PMC11416920.
- Thiyagarajan R, Zhang L, Glover OD, Kwack KH, Ahmed S, Murray E, Yellapu NK, Bard J, Seldeen KL, Rosario SR, Troen BR, Kirkwood KL. Age-related increase of CD38 directs osteoclastogenic potential of monocytic myeloid-derived suppressor cells through mitochondrial dysfunction in male mice. Aging Cell. 2024 Aug 23:e14298. doi: 10.1111/acel.14298. Epub ahead of print. PMID: 39180173.
- Vick LV, Rosario S, Riess JW, Canter RJ, Mukherjee S, Monjazeb AM, Murphy WJ. Potential roles of sex-linked differences in obesity and cancer immunotherapy: revisiting the obesity paradox. NPJ Metab Health Dis. 2024;2(1):5. doi: 10.1038/s44324-024-00007-4. Epub 2024 May 23. PMID: 38800540; PMCID: PMC11116109.
- Oturkar CC, Rosario SR, Hutson AD, Groman A, Edge SB, Morrison CD, Swetzig WM, Wang J, Park JH, Kaipparettu BA, Singh PK, Kumar S, Cappuccino HH, Ranjan M, Adjei A, Ghasemi M, Goey AKL, Kulkarni S, Das GM. ESR1 and p53 interactome alteration defines mechanisms of tamoxifen response in luminal breast cancer. iScience. 2024 May 15;27(6):109995. doi: 10.1016/j.isci.2024.109995. PMID: 38868185; PMCID: PMC11166704.
- Boland PM, Ebos JML, Attwood K, Mastri M, Fountzilas C, Iyer RV, Banker C, Goey AKL, Bies R, Ma WW, Fakih M. A phase I/II study of nintedanib and capecitabine for refractory metastatic colorectal cancer. JNCI Cancer Spectr. 2024 Apr 30;8(3):pkae017. doi: 10.1093/jncics/pkae017. PMID: 38697618; PMCID: PMC11065487.
- Lin LH, Ghasemi M, Burke SM, Mavis CK, Nichols JR, Torka P, Mager DE, Hernandez-Ilizaliturri FJ, Goey AKL. Population Pharmacokinetics and Pharmacodynamics of Carfilzomib in Combination with Rituximab, Ifosfamide, Carboplatin, and Etoposide in Adult Patients with Relapsed/Refractory Diffuse Large B Cell Lymphoma. Target Oncol. 2023 Sep;18(5):685-695. doi: 10.1007/s11523-023-00992-4. Epub 2023 Aug 26. PMID: 37632592; PMCID: PMC10803178.
- Nami Yamashita, Henry Withers, Yoshihiro Morimoto, Atrayee Bhattacharya, Naoki Haratake, Tatsuaki Daimon, Atsushi Fushimi, Ayako Nakashoji, Aaron R. Thorner, Emily Isenhart, Spencer Rosario, Mark D. Long, Donald Kufe. MUC1-C integrates aerobic glycolysis with suppression of oxidative phosphorylation in triple-negative breast cancer stem cells. iScience, Volume 26, Issue 11, 2023, 108168, ISSN 2589-0042.
- Rosario, S.R.; Dong, B.; Zhang, Y.; Hsiao, H.-H.; Isenhart, E.; Wang, J.; Siegel, E.M.; Monjazeb, A.M.; Owen, D.H.; Dey, P.; et al. Metabolic Dysregulation Explains the Diverse Impacts of Obesity in Males and Females with Gastrointestinal Cancers. Int. J. Mol. Sci. 2023, 24, 10847.
- Ramakrishnan S, Kittles RA, Huss WJ, Wang J, Attwood K, Woloszynska A. Serum Androgen Metabolites Correlate with Clinical Variables in African and European American Men with Localized, Therapy Naïve Prostate Cancer. Metabolites. 2023 Feb 16;13(2):284. doi: 10.3390/metabo13020284. PMID: 36837903; PMCID: PMC9962438.
- Rosario SR, Jacobi JJ, Long MD, Affronti HC, Rowsam AM, Smiraglia DJ. JAZF1: A Metabolic Regulator of Sensitivity to a Polyamine-Targeted Therapy. Mol Cancer Res. 2023 Jan 3;21(1):24-35. doi: 10.1158/1541-7786.MCR-22-0316. PMID: 36166196; PMCID: PMC9808368.
- Dai T, Rosario SR, Katsuta E, Sawant Dessai A, Paterson EJ, Novickis AT, Cortes Gomez E, Zhu B, Liu S, Wang H, Abrams SI, Seshadri M, Bshara W, Dasgupta S. Hypoxic activation of PFKFB4 in breast tumor microenvironment shapes metabolic and cellular plasticity to accentuate metastatic competence. Cell Rep. 2022 Dec 6;41(10):111756. doi: 10.1016/j.celrep.2022.111756. PMID: 36476868; PMCID: PMC9807018.
- Rosario SR, Smith RJ Jr, Patnaik SK, Liu S, Barbi J, Yendamuri S. Altered acetyl-CoA metabolism presents a new potential immunotherapy target in the obese lung microenvironment. Cancer Metab. 2022 Oct 26;10(1):17. doi: 10.1186/s40170-022-00292-x. PMID: 36289552; PMCID: PMC9598035.
- Jin R, Forbes C, Miller NL, Strand D, Case T, Cates JM, Kim HH, Wages P, Porter NA, Mantione KM, Burke S, Mohler JL, Matusik RJ. Glucocorticoids are induced while dihydrotestosterone levels are suppressed in 5-alpha reductase inhibitor treated human benign prostate hyperplasia patients. Prostate. 2022 Oct;82(14):1378-1388. doi: 10.1002/pros.24410. Epub 2022 Jul 12. PMID: 35821619; PMCID: PMC9427722.
- Fountzilas C, Adjei A, Opyrchal M, Evans R, Ghasemi M, Attwood K, Groman A, Bshara W, Goey AKL, Wilton J, Ma WW, Iyer R. A phase 1 study of the ALK inhibitor ceritinib in combination with gemcitabine-based chemotherapy in patients with advanced solid tumors. Int J Cancer. 2021 Dec 15;149(12):2063-2074. doi: 10.1002/ijc.33754. PMID: 34319586.
- Harding JJ, Kelley RK, Tan B, Capanu M, Do GK, Shia J, Chou JF, Ferrer CS, Boussayoud C, Muenkel K, Yarmohammadi H, El Dika I, Khalil DN, Ruiz C, Rodriguez-Lee M, Kuhn P, Wilton J, Iyer R, Abou-Alfa GK. Phase Ib Study of Enzalutamide with or Without Sorafenib in Patients with Advanced Hepatocellular Carcinoma. Oncologist. 2020 Dec;25(12):e1825-e1836. doi: 10.1634/theoncologist.2020-0521. PMID: 32548867.
- Iyer RV, Konda B, Fountzilas C, Mukherjee S, Owen D, Attwood K, Wang C, Maguire O, Minderman H, Suffren SA, Hicks K, Wilton J, Bies R, Casucci D, Reidy-Lagunes D, Shah M. Multicenter phase 2 trial of nintedanib in advanced nonpancreatic neuroendocrine tumors. Cancer. 2020 Aug 15;126(16):3689-3697. doi: 10.1002/cncr.32994. PMID: 32525561.
- Fountzilas C, Gupta M, Lee S, Krishnamurthi S, Estfan B, Wang K, Attwood K, Wilton J, Bies R, Bshara W, Iyer R. A multicentre phase 1b/2 study of tivozanib in patients with advanced inoperable hepatocellular carcinoma. Br J Cancer. 2020 Mar;122(7):963-970. doi: 10.1038/s41416-020-0737-6. PMCID: PMC7109127.
- Affronti HC, Rowsam A, Pellerite AJ, Rosario S, Long M, Jacobi J, Bianchi-Smiraglia A, Boerlin CS, Gillard B, Karasik E, Foster B, Moser M, Wilton J, Attwood K, Nikiforov MA, Azabdaftari G, Pili R, Phillips JG, Casero RA Jr, Smiraglia D. Pharmacological polyamine catabolism upregulation with methionine salvage pathway inhibition as an effective prostate cancer therapy. Nat Commun. 2020 Jan 7;11(1):52. doi 10.1038/s41467-019-13950-4. PMCID: PMC6946658.
- Kamoun WS, Kirpotin DB, Huang ZR, Tipparaju SK, Noble CO, Hayes ME, Luus L, Koshkaryev A, Kim J, Olivier K, Kornaga T, Oyama S, Askoxylakis V, Pien C, Kuesters G, Dumont N, Lugovskoy AA, Schihl SA, Wilton JH, Geddie ML, Suchy J, Grabow S, Kohli N, Reynolds CP, Blaydes R, Zhou Y, Sawyer AJ, Marks JD, Drummond DC. Antitumour activity and tolerability of an EphA2-targeted nanotherapeutic in multiple mouse models. Nat Biomed Eng. 2019 Apr;3(4):264-280. doi: 10.1038/s41551-019-0385-4. PMID: 30952988.
- Ma WW, Xie H, Fetterly G, Pitzonka L, Whitworth A, LeVea C, Wilton J, Mantione K, Schihl S, Dy GK, Boland P, Iyer R, Tan W, Brady W, Straubinger RM, Adjei AA. A Phase Ib Study of the FGFR/VEGFR Inhibitor Dovitinib With Gemcitabine and Capecitabine in Advanced Solid Tumor and Pancreatic Cancer Patients. Am J Clin Oncol. 2019 Feb;42(2):184-189. doi: 10.1097/COC.0000000000000492 PMCID: PMC6345595
- Cook SF, Fiandalo MV, Watt DS, Wu Y, Mohler JL, Bies RR. Mathematical modeling of intracrine androgen metabolism in prostate cancer: Methodological aspects. Prostate. 2018 Jun 25. doi: 10.1002/pros.23665. PMID: 29938815
- Hess TA, Drinkhouse ME, Prey JD, Miller JM, Fettig AA, Carberry CA, Brenn SH, Bailey DB. Analysis of platinum content in biodegradable carboplatin-impregnated beads and retrospective assessment of tolerability for intralesional use of the beads in dogs following excision of subcutaneous sarcomas: 29 cases (2011-2014). J Am Vet Med Assoc. 2018 Feb 15;252(4):448-456. doi: 10.2460/javma.252.4.448 PMID: 29393745
- Fiandalo MV, Wilton JH, Mantione KM, Wrzosek C, Attwood KM, Wu Y, Mohler JL. Serum-free complete medium, an alternative medium to mimic androgen deprivation in human prostate cancer cell line models. Prostate. 2018 Feb;78(3):213-221. doi: 10.1002/pros.23459. Epub 2017 Dec 1 PMCID: PMC5768451
- Fiandalo MV, Stocking JJ, Pop EA, Wilton JH, Mantione KM, Li Y, Attwood KM, Azabdaftari G, Wu Y, Watt DS, Wilson EM, Mohler JL. Inhibition of dihydrotestosterone synthesis in prostate cancer by combined frontdoor and backdoor pathway blockade. Oncotarget. 2018 Jan 10;9(13):11227-11242. doi: 10.18632/oncotarget.24107. eCollection 2018 Feb 16 PMCID: PMC5834294
- Vicky C. Chang, Michelle Cotterchio, Beatrice A. Boucher, David J. A. Jenkins, Lucia Mirea, Susan E. McCann & Lilian U. Thompson (2018) Effect of Dietary Flaxseed Intake on Circulating Sex Hormone Levels among Postmenopausal Women: A Randomized Controlled Intervention Trial, Nutrition and Cancer, DOI: 10.1080/01635581.2018.1516789
Tools and equipment
BMPK instrumentation
- Sciex 7500 Triple quadrupole (ESI/APCI-LC/MS/MS)
- Agilent 6545B LC/Q-TOF MS system
- Sciex 5500 QTRAP triple quadrupole (ESI/APCI-LC/MS/MS)
- Sciex 5500 triple quadrupole (ESI/APCI-LC/MS/MS)
- Thermo Scientific TSQ Vantage triple quadrupole (ESI/APCI-LC/MS/MS)
- Sciex API3000 triple quadrupole (ESI LC/MS/MS)
BMPK modeling software
- Phoenix WinNonlin: Non-compartmental and compartmental modeling
- NONMEM: Nonlinear mixed effect modeling software for population analysis to determine sources of variability in the PK and PD of drugs
- ADAPT 5 and S-ADAPT: individual compartmental PK and PK/PD modeling
- SAS 9.3: used to manage and clean databases, and create graphics and tables
Fees
Collaborative interaction is essential to achieve optimal experimental design and outcomes. As a client of BMPK, we will arrange a one-on-one consultation with you to review your experimental design, method development needs, sample analysis strategy, and results interpretation in order to best meet your study aims and objectives. Over the course of the study, we will work closely with you to ensure that you receive the most pertinent and reliable outcomes possible. As a result, pricing varies with experimental design, turnaround time, the number of analytes as well as the number of samples.
Investigators interested in the use of the BMPK Shared Resource should contact Joshua Prey to discuss scheduling, shared resource procedures and pricing.
Contact usMeet our team
Joshua Prey, MS
Research Project Administrator
Phone: 716-845-1300, ext. 3313
Email: Joshua.Prey@RoswellPark.org
Location and hours
Roswell Park Comprehensive Cancer CenterBioanalytics, Metabolomics & Pharmacokinetics Shared Resource
Center for Genetics and Pharmacology, Room L1-140
Elm and Carlton Streets
Buffalo, New York 14263
Monday - Friday, 8 a.m. - 5 p.m.
BMPK is partially funded by NCI P30CA16056. Authors should cite the core grant in the acknowledgment section of the publication if data that was generated by BMPK is used in the publication. Two copies of the publication, acknowledging the core grant, should be submitted to the facility at Elm and Carlton Streets, Buffalo NY, 14263.
For BMPK facility data, please click here.