The roles of IL-17/ILC-17.1 and CEP-1/p53 in development, aging, and dormancy
During metazoan development, cytokines, growth factors, and other small molecules license the acquisition of nutrients and the activation of anabolic programs in cells to promote their proliferation, growth, differentiation, and defense pathways.
Yet, how cell fate decisions and energy requirements of different cells and tissues are coordinated with nutrient availability remains poorly understood.
Discoveries
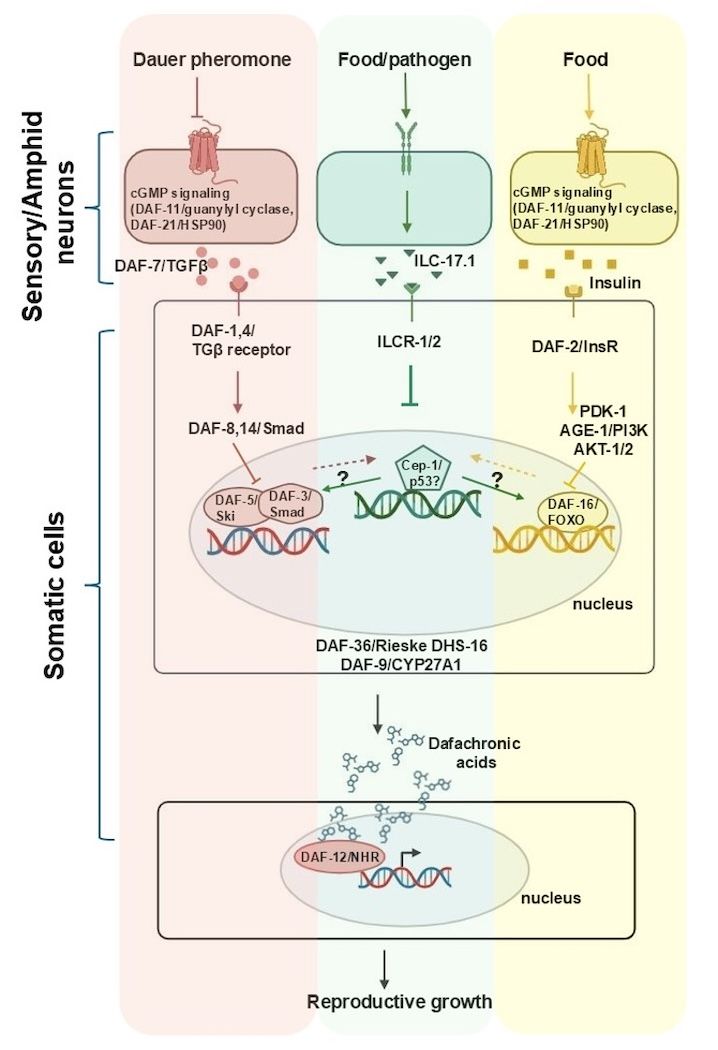
We have recently made the exciting discovery that in the genetically tractable model organism C. elegans, the cytokine IL-17 ortholog ILC-17.1 is released from sensory neurons in response to food availability and modulates the division, growth, and metabolic activities of other cells through its control over the DNA-damage-independent function of the tumor suppressor p53 ortholog, CEP-1.
Specifically, ILC-17.1 release is required to allow larvae to grow continuously into reproductive adults. The absence of ILC-17.1- signaling pathway components (either ligand or receptor) activates p53/CEP-1 and triggers a systemic change in morphology and physiology, causing larvae to arrest development in a dormant, stress-resistant state, dauer.
The ILC-17.1/p53/CEP-1 axis:
- Acts genetically upstream of the other, well-characterized C. elegans dauer-activating pathways, FOXO [DAF-16], TGFβ [DAF-3/SMAD-DAF-5/Ski], and steroid hormone receptors;
- Controls the reversible upregulation of cell cycle inhibitors;
- Modulates glucose utilization;
- Alters respiration, the expression of mitochondrial OXPHOS genes, phosphofrucktokinase, and cytochrome c; and
- Alters the expression of targets of p38 MAPK innate immune signaling
Based on these observations, we hypothesize that the ancestral role of IL-17s, as displayed by the C. elegans ILC-17.1, is to coordinate resource allocation with cell fate regulation by CEP-1/p53 to license anabolic growth.
Open questions
The ability to temporarily arrest cell cycle progression, pause or delay growth, and enter a hypometabolic state is a key feature of dormancy and is a valuable program used by cells and organisms to tide over harsh conditions.
We are now interested in:
- Understanding the mechanisms by which the nervous system senses and signals stress.
- Understanding how cells and tissues coordinate their growth and cell cycle arrest to result in organ-wide tissue remodeling and dauer arrest.
- Exploring parallels between C. elegans dauers and dormant cancer cells.
See the science
Godthi A, Min S, Das S, Cruz-Corchado J, Deonarine A, Misel-Wuchter K, Issuree PD, Prahlad V. Neuronal IL-17 controls Caenorhabditis elegans developmental diapause through CEP-1/p53. Proc Natl Acad Sci U S A. 2024 Mar 19;121(12):e2315248121. doi: 10.1073/pnas.2315248121.
Connect with the Prahlad Lab
Email Dr. PrahladGitHub
Department of Cell Stress Biology
Roswell Park Comprehensive Cancer Center
Elm and Carlton Streets
Buffalo, NY 14263