Funded and ongoing projects
Investigating how stress-induced changes in maternal serotonin affect offspring development and stress resilience
R01 | National Institute of Mental Health | Principal Investigator
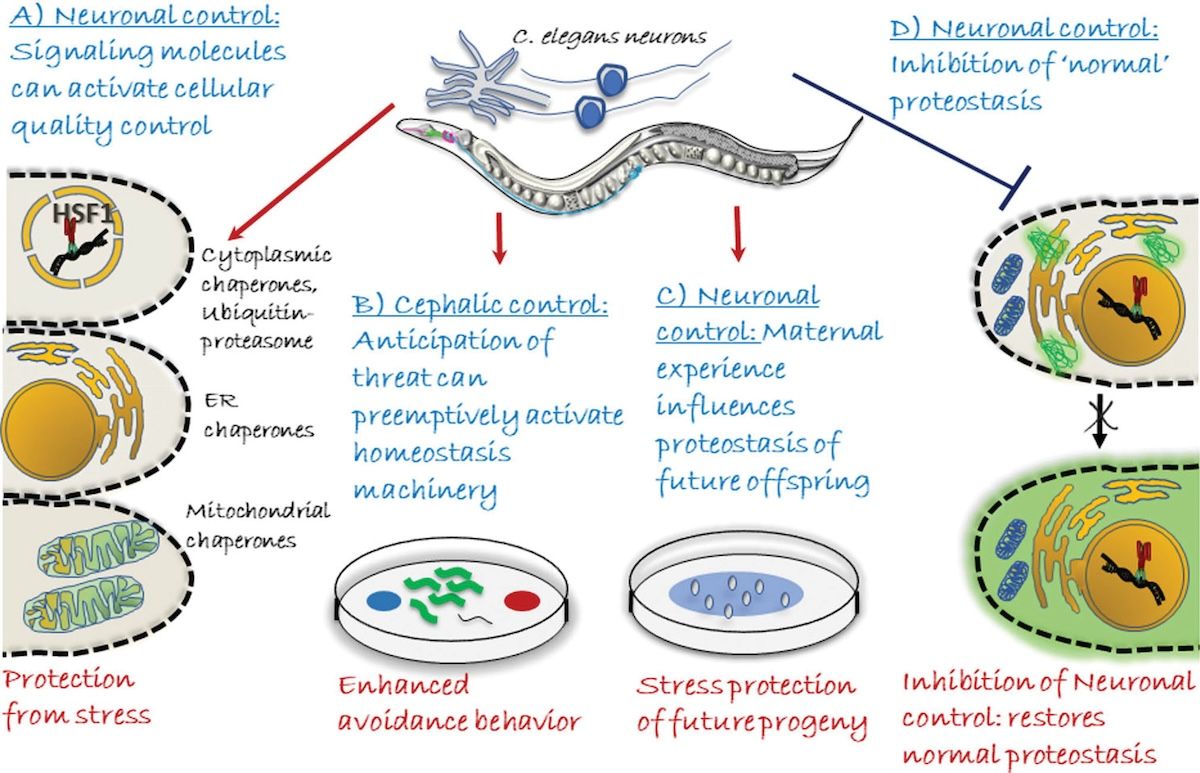
We have discovered that the stress-induced release of serotonin from maternal neurons in C. elegans enables the transcription factor, HSF1 to modify chromatin in pre-fertilized oocytes through a piRNA-mediated chromatin silencing mechanism and thereby influences offspring development and stress-resilience.
We seek to understand the molecular mechanisms by which this occurs:
- Aim 1: Identify the neural bases and signaling mechanisms of the serotonergic defense survival circuit in the parent.
- Aim 2: We will identify the epigenetic changes in pre-fertilized germ cells caused by maternal serotonin, and understand how they impact offspring neurodevelopment, behavior, and stress resilience.
Project Details: R01 MH126282Related publications
Role of serotonergic-activation of heat shock transcription factor in the regulation of age-related protein misfolding and toxicity in mammalian systems
R21 | National Institute on Aging | Contact PI/Project Leader
We have discovered that the conserved neuromodulator, serotonin, traditionally known for its roles in neuropsychiatric diseases, can activate a conserved gene expression program in mammalian neurons that counteracts the disruption of protein homeostasis.
We are testing the hypothesis that modulating serotonergic signaling through 5-HT4R can protect against proteinopathies in a mouse model of Huntington's Disease by activating HSF1 and intend to dissect the intracellular mechanisms underlying such protection.
If successful, our studies will establish a rational basis for exploring the role for 5-HT4R agonists in the protection against devastating neurodegenerative diseases of aging and provide evidence that serotonin-dependent changes in motivational state can modulate proteinopathies.
Co-Principal Investigator: Rocio Gomez-Pastor, PhD, University of Minnesota
Investigating how intestinal innate immunity confers neuroprotection using C. elegans
R01 | National Institute on Aging | Contact PI/Project Leader
We have discovered that the activation of the innate immune response in intestinal cells of C. elegans can modulate mitochondrial homeostasis in neurons.
We seek to understand how this occurs and how the disruption of innate immunity in peripheral tissue, known to occur during aging, may impact mitochondrial homeostasis in neurons and mediate neurodegeneration:
- Aim 1: Examine the role of the innate immune response in the maintenance of neurons upon Complex I dysfunction.
- Aim 2: Identify the immune mediators in the gut that affect neuronal health.
- Aim 3: Examine the role of peripheral proteotoxic antigens in disrupting immune signaling, leading to the accumulation of dysfunctional neuronal mitochondria.
Project Details: R01 AG060616Related publications
Completed research projects
Uncovering how serotonergic signaling non-autonomously regulates protein homeostasis
R01 | National Institute on Aging | Principal Investigator
We have discovered that activation of conserved neurohormonal mechanisms can trigger the cells' own protective responses to suppress misfolding and aggregation of disease related proteins. Our studies will elucidate, in-depth, for the first time, a mechanism by which the nervous system controls cellular protective mechanisms that can suppress proteinopathies.
Hypothesis: Thermosensory-induced release of 5-HT activates adaptive cellular stress responses that protect protein homeostasis.
- Aim 1: How do thermosensory (AFD) neurons elicit 5-HT release from serotonergic neurons?
- Aim 2: What inter-tissue stress signaling pathways are activated by 5-HT in responsive cells?
Project Details: R01 AG050653Related publications
Uncovering non-autonomous mechanisms of control over translational attenuation during heat shock in the metazoan C. elegans
R21 | National Institute of Neurological Disorders and Stroke | Principal Investigator
We have recently discovered that conserved neurohormonal signaling pathways that trigger chaperone upregulation during heat stress are required for the systemic attenuation of translation upon heat shock. We are investigating the mechanisms by which neurohormonal signaling controls eIF2α phosphorylation upon stress and ask whether neuronal signaling acts to integrate transcriptional and translational responses to stress across cells of an organism.
Specifically, we are testing:
- How translation attenuation is coupled to neuronal serotonin release
- Whether translation attenuation is coordinated with HSP upregulation through neuronal 5-HT release
Project Details: R21 NS097942Cell Sci. 2018 Nov 21;131(22)
Connect with the Prahlad Lab
Email Dr. PrahladGitHub
Department of Cell Stress Biology
Roswell Park Comprehensive Cancer Center
Elm and Carlton Streets
Buffalo, NY 14263