Control of the heat shock transcription factor in cells of a metazoan
HSF1 is a universal stress-responsive transcription factor activated by almost all environmental and physiologically relevant stressors and defends the cell from protein misfolding and aggregation. Across species, HSF1 is necessary for survival upon stress.
The model for HSF1 activation that has dominated the field for 30 years and is supported by numerous elegant studies in isolated cells and unicellular organisms like yeast, is that HSF1 is activated through feedback control by increases in aberrant protein conformations that accrue upon proteotoxic stress exposure (Hartl FU, 2011; Lindquist, 1986).
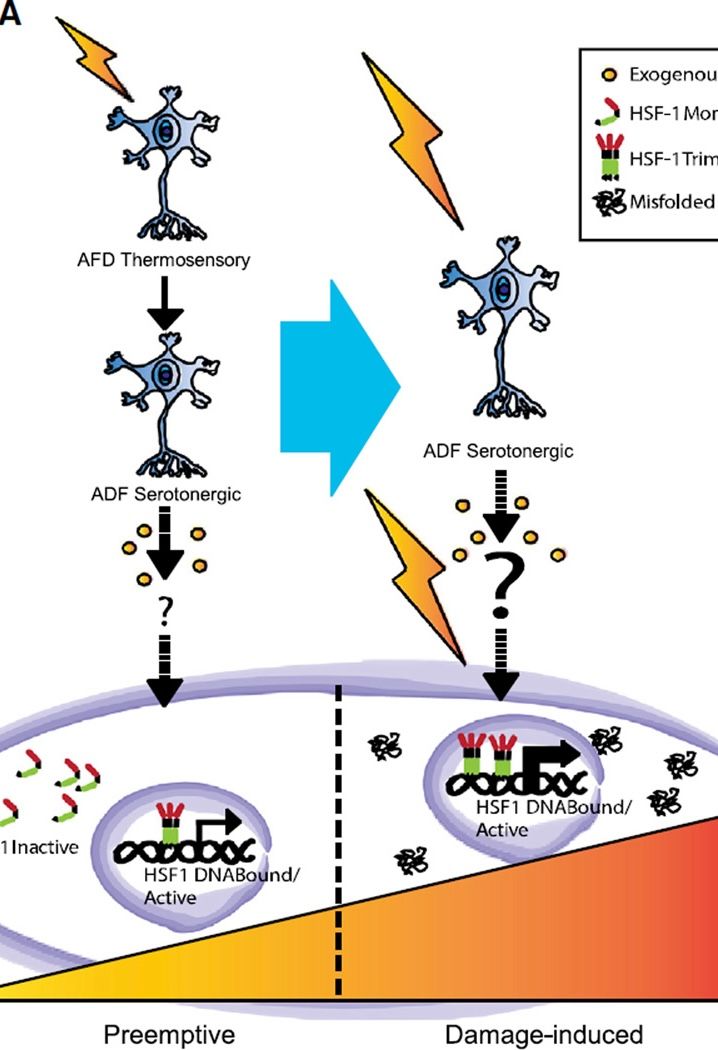
In agreement with this model, HSF1 is activated in all tissues of metazoans such as Caenorhabditis elegans, albeit to varying degrees, when the organism is exposed to environmental stressors, like elevated temperatures, that destabilize proteins.
However, notwithstanding the model whereby HSF1 is activated by protein misfolding, HSF1 is not consistently activated in response to protein aggregation in diseases of protein aggregation. This is true for animal models of disease or in brain tissue from humans affected by neurodegenerative proteinopathies. On the other hand, HSF1 is activated in almost all cancers, where it is necessary to promote malignancy, metastasis, invasion, proliferation, and cancer cell metabolism.
How HSF1 is regulated to have opposing transcriptional programs in cancer and neurodegenerative diseases is unknown. In mammals, several reasons have been proposed for this failure in the expected activation of HSF1 in neurodegenerative disease, or its increased activation in cancer; however, none of these reasons comprehensively explain the dyregulation of HSF1 that occurs in disease.
By examining how cells within the multicellular organism C. elegans control the activity of HSF1 during normal physiology, in response to stress, and protein misfolding, we are beginning to uncover complex layers of control over HSF1 that shed light on this problem.
Discoveries
- HSF1 is controlled by the nervous system of C. elegans
- HSF1 can be activated by the perception of a threat, before actual encounter with the threat
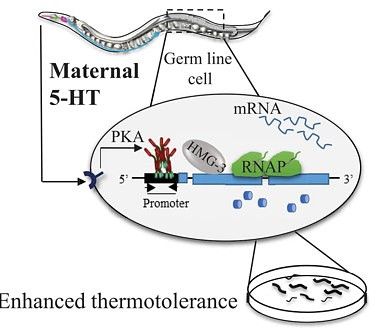
- One of the signaling molecules that activates HSF1 is serotonin
- Serotonin acts through conserved receptor-mediated signal transduction pathways (GPCR) to activate Protein Kinase A (PKS), which phosphorylates HSF1
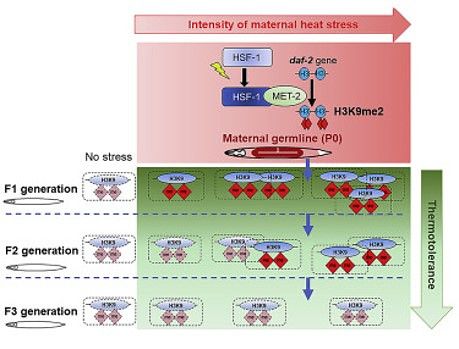
- HSF1 activation generates a transgenerational epigenetic memory of stress exposure in C. elegans
Open questions
- Is HSF1 activation also controlled by the nervous system in mammals?
- How does serotonin activate HSF1? Does it do so in all cells and tissues? Is HSF1 activated during memory and learning through serotonergic circuits? If so, when and how? What are the consequences? Can this signaling pathway be leveraged for intervention in disease?
- What is the effect of serotonin over HSF1 in cancer cells and tumor progression?
- What neuroendocrine molecules, besides serotonin, can activate HSF1? Are they conserved?
- What epigenetic changes are triggered by HSF1 activation, and how are they maintained across generations of the population to retain a ‘memory’ of prior stress exposure? Do these similar HSF1-dependent mechanisms function in mammalian/cancer cells?
- What are the physiological roles of HSF1 in animals? (during fever, in restraint stress, during hyperthermia, learned helplessness, neurodevelopment, learning and memory), and can we link those roles to disease?
- Can we leverage mechanisms underlying neuronal control over HSF1 to intervene in cancer or neurodegenerative diseases?
Connect with the Prahlad Lab
Email Dr. PrahladGitHub
Department of Cell Stress Biology
Roswell Park Comprehensive Cancer Center
Elm and Carlton Streets
Buffalo, NY 14263