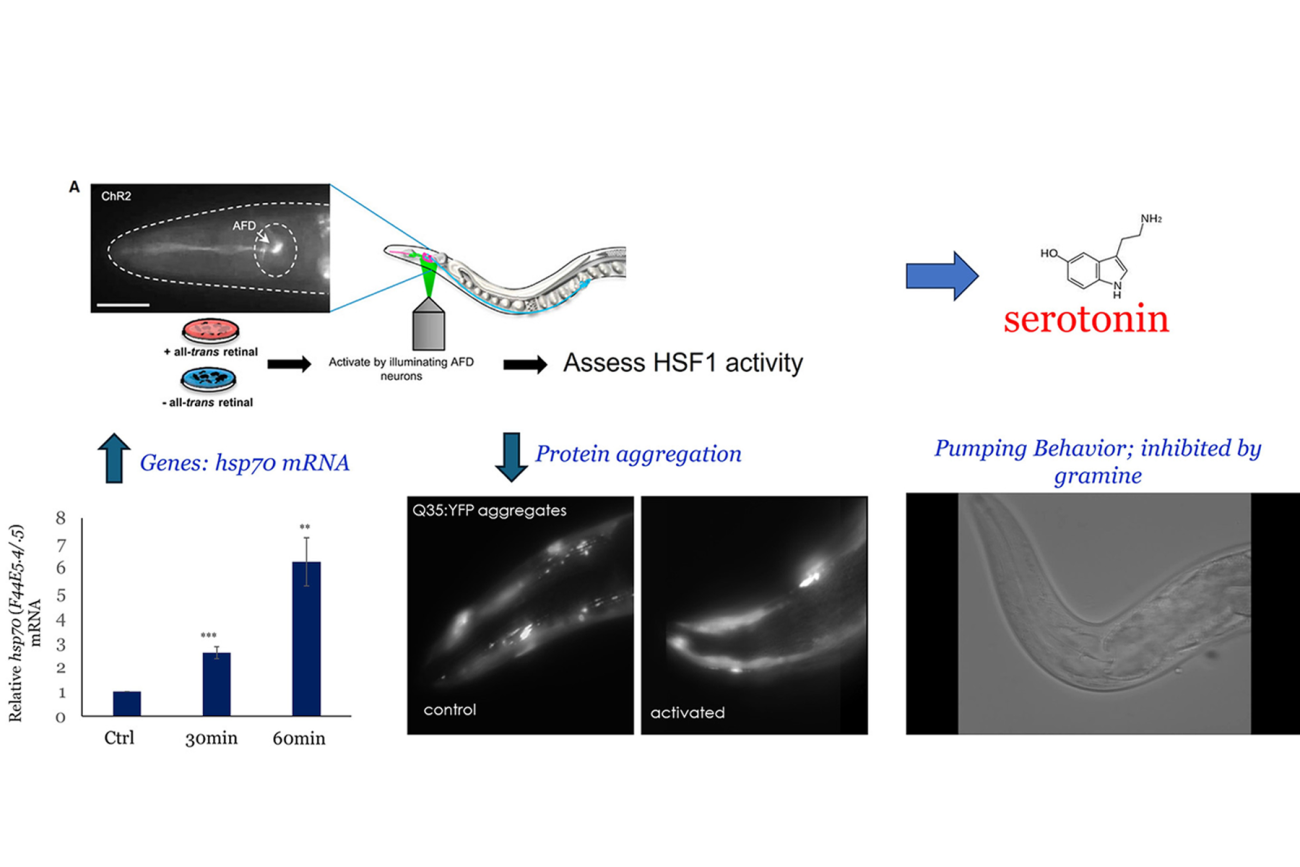
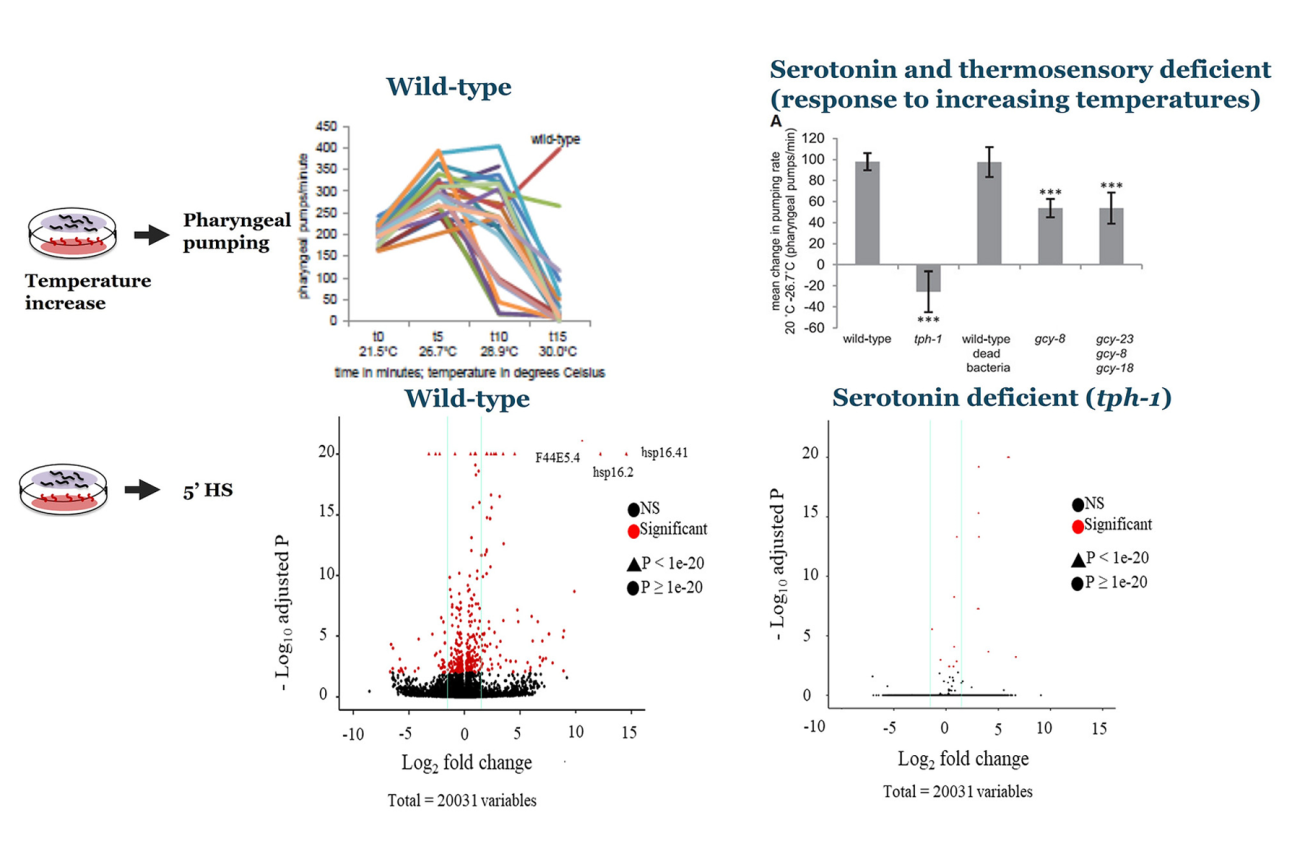
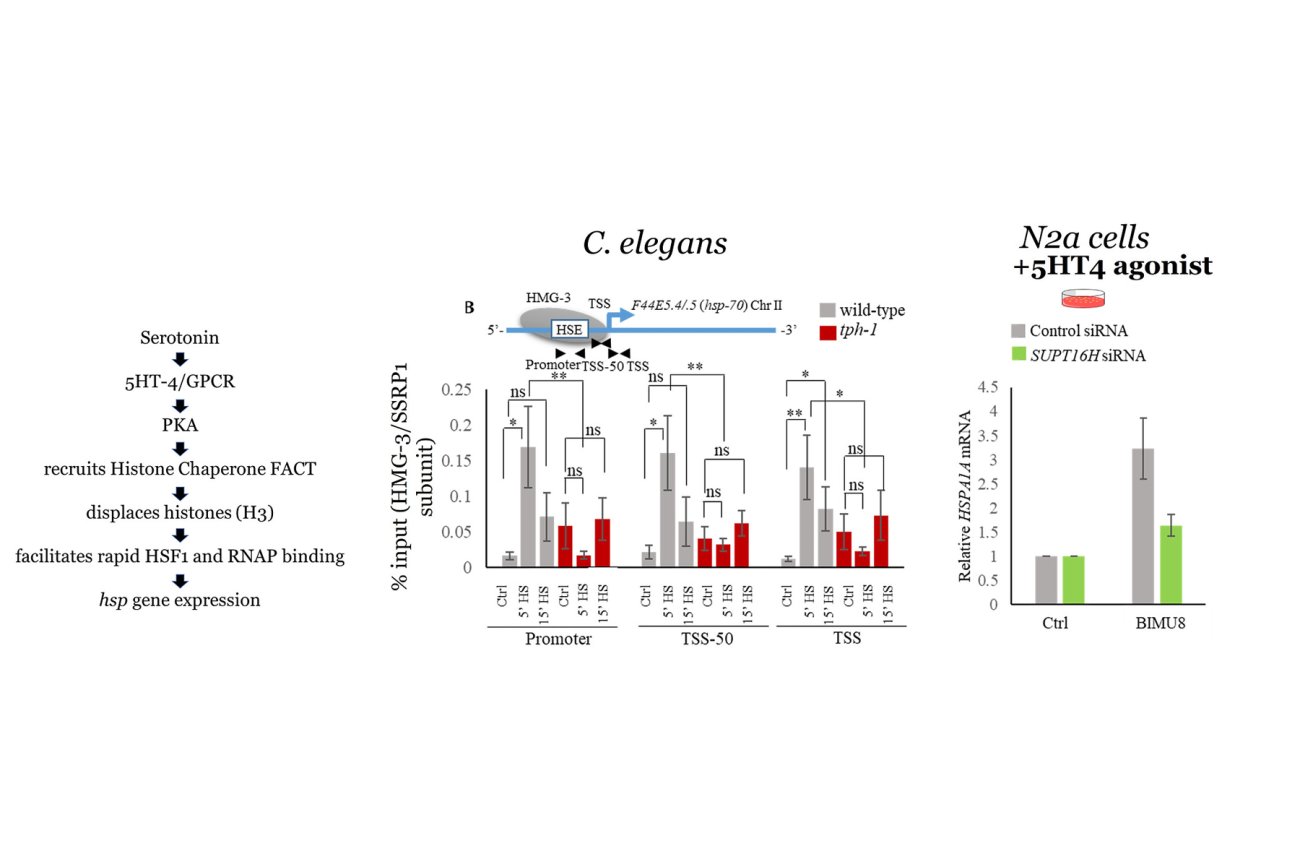
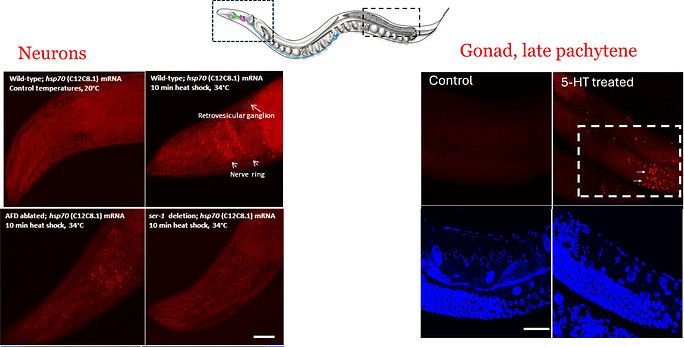
Neural control of cell stress response pathways and transcription factors
The nervous system controls somatic physiology, development, and tissue regeneration and also drives aging, and progression of diseases such as cancer. Yet, the governing principles, rules of interaction between nerves and non-neural tissues, and mechanisms remain largely unknown.
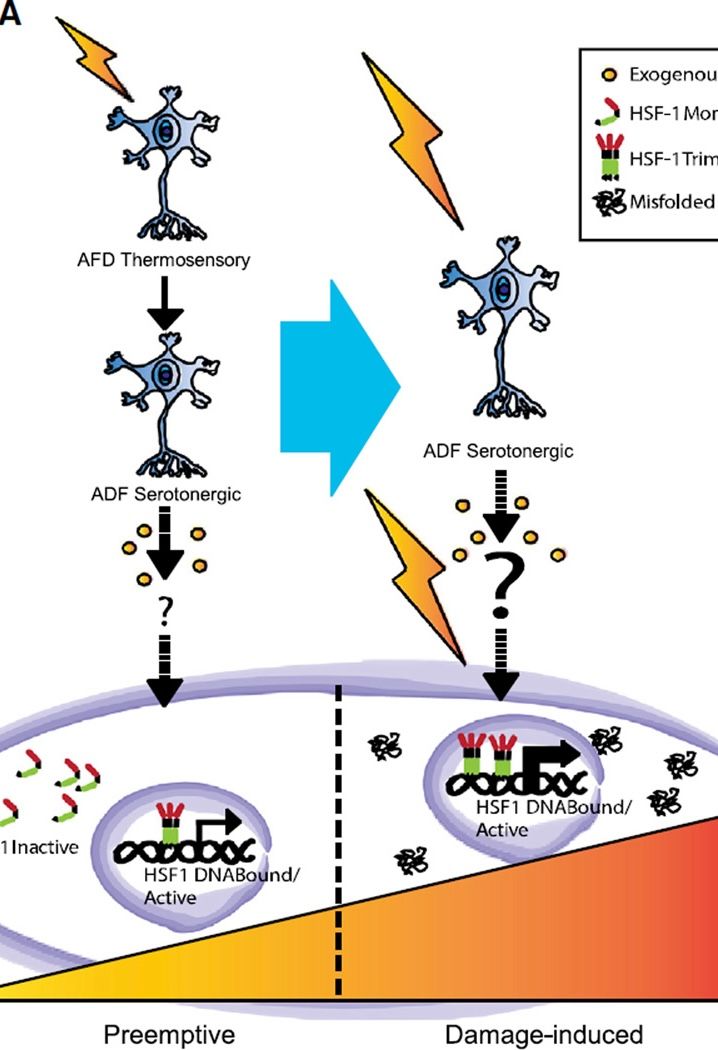
Over a decade ago, we discovered that the Caenorhabditis elegans nervous system controls when and whether other cells within the body of the animal activate their heat-shock response. This was surprising since the so-called ‘heat-shock response’ is a defense program against protein misfolding fundamental to all functions of a cell, and long considered to be controlled autonomously, by the cell in which misfolding occurred.
My lab has since investigated how neuronal signals control conserved stress-responsive transcription programs of other cells, both neuronal and non-neural. We have made seminal contributions in this area.
Click for larger image
An emerging theme
Our studies demonstrate that in a metazoan, neuronal networks repress – and subsequently de-repress – stress response pathways that can be activated autonomously in isolated cells, thereby co-opting these defense programs of cells to cephalic control.
Our working model is that cell stress programs can be:
- Activated pre-emptively, by perceived threats, before damage occurs; and
- Activated unnecessarily even when the ‘real’ danger has subsided
What we’ve learned
Cephalic control over the transcription factor HSF1 is sufficient to:
- Upregulate molecular chaperones;
- Suppress protein misfolding; and
- Establish HSF1-induced durable epigenetic changes in cells that receive the neuronal signal
We’ve also learned that in C. elegans persist over two generations, through epigenetic mechanisms also conserved in mammalian cells.
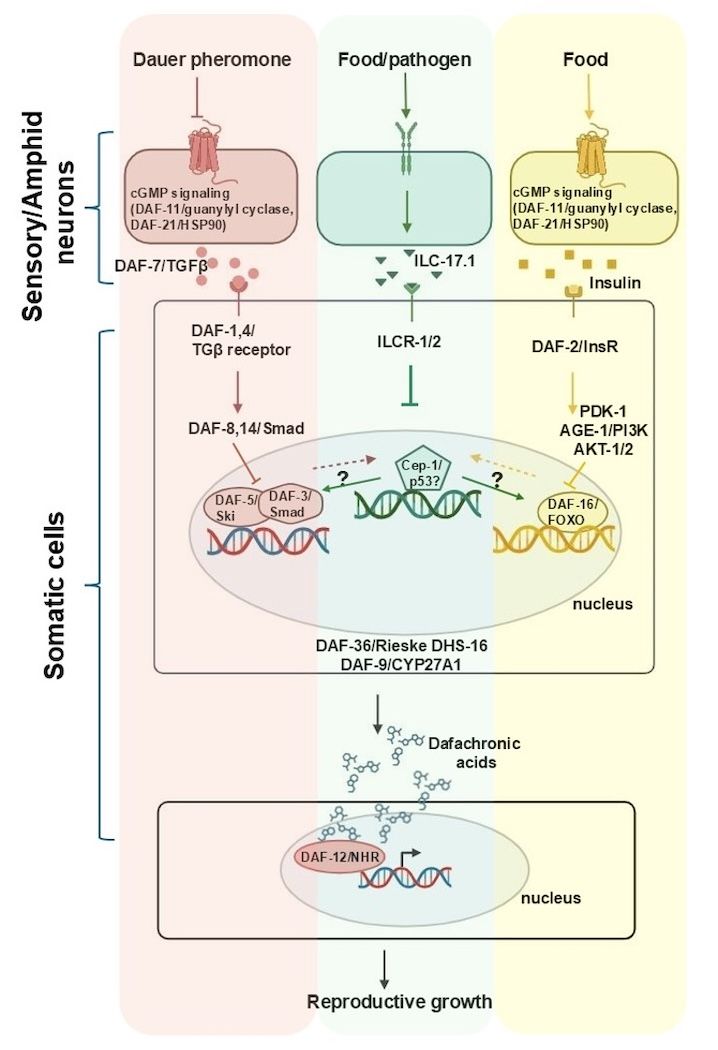
Recently, we found another important stress response pathway is also controlled by C. elegans neurons:
- Control the DNA-damage independent activation of the tumor suppressor p53-ortholog in larval pluripotent blast cells through the neuronal release of the C. elegans IL-17 cytokine; and
- Determine whether larvae arrest in dormancy or grow to reproductive adults.
What we’re learning
These discoveries have led to the establishment of two major research projects in the lab, where we use the metazoan model C. elegans, mammalian cell culture, and mouse models to address questions.
Project 1: Control of the heat shock transcription factor in cells of a metazoan
Project 2: The roles of IL-17/ILC-17.1 and CEP-1/p53 in development, aging, and dormancy
Connect with the Prahlad Lab
Email Dr. PrahladGitHub
Department of Cell Stress Biology
Roswell Park Comprehensive Cancer Center
Elm and Carlton Streets
Buffalo, NY 14263