The role of FACT in normal development and cancer
More and more evidence demonstrates extensive reorganization of chromatin in cancer cells. In trying to identify factors responsible for this reorganization, we established that an increase in the expression and activity of histone chaperone FACT is a critical event in malignant transformation.
Based on literature, FACT interacts with different components of nucleosomes to provide access of transcription, replication and DNA repair machineries to nucleosomal DNA, and at the same time to ensure nucleosome stability. With this quite basal function, FACT is surprisingly selectively expressed in mammals (Garcia H. et al, 2011).
The highest level of FACT is observed during early embryonic development. Postnatal expression of both FACT subunits is limited to subpopulations of cells of lymphoid, reproductive and few other tissues. However, their expression is reinstated in multiple types of tumors.
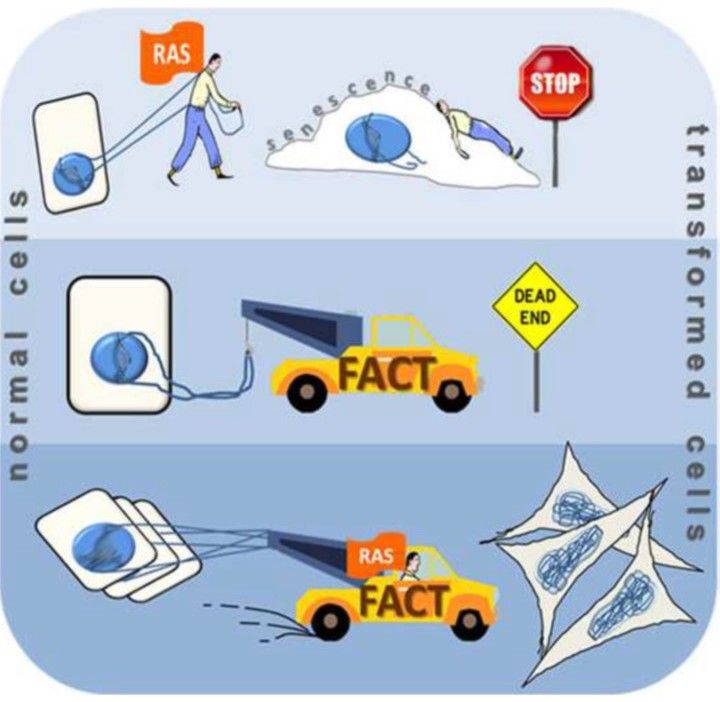
Moreover, expression of FACT is essential for growth and survival of tumor, but not normal cells. Yet FACT is not mutated or amplified in tumors and its overexpression does not drive malignant transformation in the absence of oncogene.
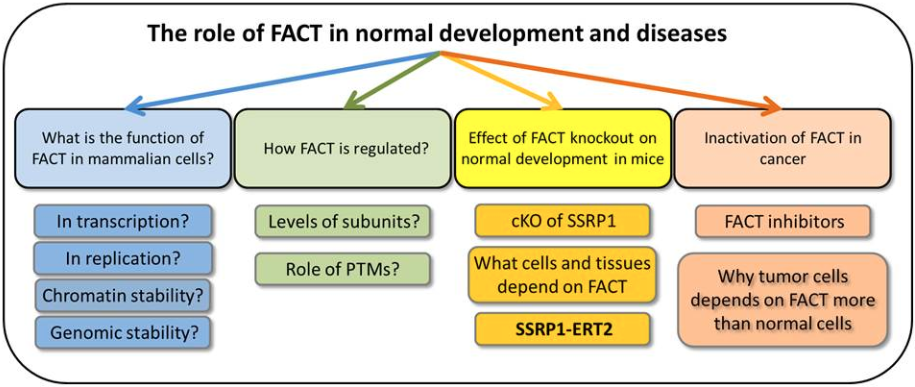
Why do stem and malignant tumor cells love to have a lot of FACT?
Embryonic stem cells (ESCs) and malignant tumor cells are the highest FACT expressing cells, many folds higher than any other cells in mammals. Moreover, these two categories of cells are extremely sensitive to FACT inhibition; both types of cells die if FACT is depleted (Cao S. et al, 2003, Garcia H. et al, 2013), while viability of normal cells with lower expression of FACT is not compromised.
Tumor cells with moderate amount of FACT slow down or stop proliferation in response to FACT knockdown (Fleyshman D. etal, 2017). We are now trying to understand what exactly FACT does in ESCs and tumor cells.
There are several possible scenarios based on known FACT activities observed in cell-free systems and model organisms. FACT may ensure a high rate of replication and transcription needed for these categories of cells; it may reduce the level of replication stress. It may also be critical for maintaining chromatin integrity via prevention of nucleosome loss when transcription and replication are too high.
We are testing all these possibilities to understand what makes ESCs and malignant tumor cells so uniquely dependent on FACT.
How is FACT level regulated and what drives it up in cancer?
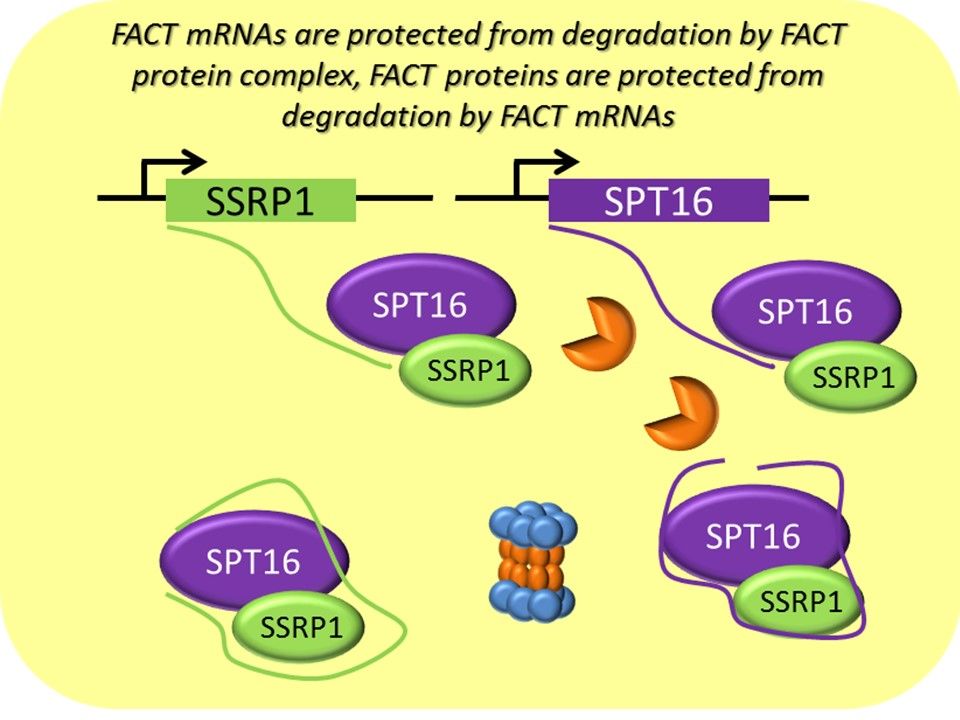
FACT consists of two subunits: SSRP1 and SPT16. They form complex and outside of the complex both subunits are very unstable (Safina A. et al, 2013). However, a puzzle that we’ve observed since we started working with FACT is that when we inhibit expression of SSRP1 subunit with different genetic means, the protein levels of SPT16 subunits disappear quicker than of SSRP1.
We proposed and demonstrated that SSRP1 mRNA is involved in the formation and stabilization of FACT complex. But the details of this mechanism are still far from a complete understanding.
In tumors, the rate of mutations in both subunits are lower than background, suggesting that their function is essential for tumors. This is also confirmed by the high frequency of both subunits’ overexpression in multiple types of tumors.
Importantly in tumors there is poor correlation between both subunits mRNA and protein levels and increase in protein amount is more prominent, suggesting that increase of FACT in cancer is not just due to the induced expression of SSRP1 and SPT16 genes.
Our working hypothesis that FACT is mostly regulated at the level of protein stability, which depends on the presence of certain regulatory factors. We are trying to identify and to establish if it is responsible for driving up FACT levels in cancer.
Understanding this mechanism will show us how to develop FACT inhibitors for the treatment of cancer.
How does FACT select genes to assist transcription?
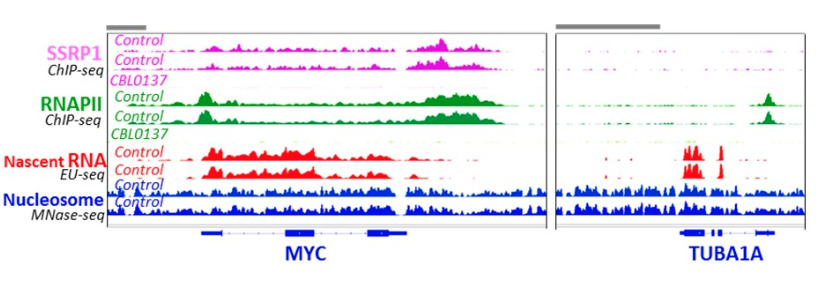
FACT distribution over genome identified using ChIP-sequencing technique is very nonrandom. FACT profile of enrichment in most cases precisely coincides with coding regions of some, but not all of expressed genes.
FACT does not have sequence specific DNA binding domain or “reader” domain to recognize histone modifications. Thus, there is no standard way for FACT to spot its targets.
We are working on the non-canonical ways which can be used by FACT to find its “job assignment.” This question is important for the understanding of another layer of regulation of transcription through the help of histone chaperones, i.e., via regulation of nucleosome stability over bodies of genes, which is completely unexplored right now.
Can FACT be safely inhibited at postnatal stage of the development and will this lead to the reduced incidence of cancer? Or maybe abolition of cancer at all?!
General knockout of SSRP1 subunit is lethal at 3.5dpc (Cao S. et al, 2003). However, low and rare expression of FACT postnatally suggested that its inactivation may not be so fatal after birth.
With this in mind we generated a mouse model of conditional knockout of SSRP1. Using these mice, we plan to define precisely the consequences for healthy tissues at different stages of mouse life FACT inactivation may have. This will help us to foresee what side effects can be expected from FACT inhibitors in clinic in adult and pediatric patients.
We also plan to estimate how effective FACT inhibiting therapy will be for different types of tumors and as cancer preventive strategy, how long it should be used and whether tumors would re-emerge after stopping the therapy.
How can we develop pharmacological inhibitor of FACT?
FACT is not an enzyme, so there is no way to inhibit its catalytic activity. FACT interacts with nucleosome via multiple binding events, and we are not aware so far which interaction is critical for FACT function in cancer.
Based on today’s state of knowledge, we decided that the best approach would be disruption of two subunits interaction, since both of them become very unstable upon this. We are perusing several paths to develop FACT inhibitors. Each path has its own obstacles.
- First, we tried to develop a cell-based readout model, which is our favorite way to look for the small molecules active in cells. However, using available chemical libraries, we so far have found no good inhibitors.
- Second, we tried to use a structure-based approach. Crystal structure of human FACT is not available, but structure of SSRP1/SPT16 binding domains is available from fungi. So, we first “humanized” FACT binding domains using sequence alignment and structure prediction tools and then tried to foresee which sites within dimerization domains of both subunits can be essential for their binding. Now we run in silico screenings and lab testing of small molecules for the inhibition of two subunits binding.
- The third approach aims to identify peptides which will be able to disrupt two subunits binding via screening of genetic libraries built from DNA fragments encoding SSRP1 and SPT16 binding domains.
Recent publications
- Goswami I, et al. FACT maintains nucleosomes during transcription and stem cell viability in adult mice. EMBO Rep. 2022 Feb 18:e53684. doi: 10.15252/embr.202153684. PMID: 35179289.
- Ehteda A, et al. Dual targeting of the epigenome via FACT complex and histone deacetylase is a potent treatment strategy for DIPG. Cell Rep. 2021 Apr 13;35(2):108994. doi: 10.1016/j.celrep.2021.108994. PMID: 33852836.
- Prendergast L, et al. Histone chaperone FACT is essential to overcome replication stress in mammalian cells. Oncogene. 2020 Jul;39(28):5124-5137. doi: 10.1038/s41388-020-1346-9. Epub 2020 Jun 12. PMID: 32533099; PMCID: PMC7343669.
- Sandlesh P, et al. Prevention of Chromatin Destabilization by FACT Is Crucial for Malignant Transformation. iScience. 2020 Jun 26;23(6):101177. doi: 10.1016/j.isci.2020.101177. Epub 2020 May 18. PMID: 32498018; PMCID: PMC7267732.
- Chang HW, et al. Histone Chaperone FACT and Curaxins: Effects on Genome Structure and Function. J Cancer Metastasis Treat. 2019;5:78. doi: 10.20517/2394-4722.2019.31. Epub 2019 Nov 29. PMID: 31853507; PMCID: PMC6919649.
Contact the Gurova Lab
Email: Katerina.Gurova@RoswellPark.org
Phone: 716-845-4760
Office location: Center for Genetics & Pharmacology (CGP) L3-301A
Lab Location: Center for Genetics & Pharmacology (CGP) L3-121 & 122
Department of Cell Stress Biology
Roswell Park Comprehensive Cancer Center
Elm and Carlton Streets
Buffalo, NY 14263