Understanding the interaction between the immune system and cancer to enhance patient outcomes
Our mission of the Tumor Immunology & Immunotherapy Program (TII) at Roswell Park Comprehensive Cancer Center aims to deepen our understanding of how the immune system can be harnessed to prevent or treat patients with all types of cancer. This mission is built on the premise the immune system can control and eradicate human cancers, and innovative strategies to engage these responses can positively impact the lives of those who have or are at risk of developing cancer.
Novel immunotherapies in TII stem from our original basic and translational science discoveries that are effectively translated into the clinic at our Cancer Center through robust partnerships with Cancer Center Support Grant members inside and outside our program, multi-institutional collaborators, exceptional shared resources and Shared Resource Directors and staff, and critical input from our Community Outreach and Engagement team.
Our goal is that TII-led innovative immuno-oncology clinical trials will continue to impact our cancer patients' longevity and quality-of-life profoundly.
Program aims
Immune-Tumor Interactions
Define key mechanisms driving tumor-intrinsic and host-derived immune responses during cancer progression.
Therapeutic Resistance and TME
Investigate mechanisms of resistance within the tumor microenvironment that impede effective antitumor immunity.
Translational Immunotherapy Innovation
Accelerate the development of novel immunotherapies and rapidly translate these discoveries into impactful clinical trials.
How can we use the body’s immune system to prevent and treat cancer?
Adoptive cellular therapy. Cancer vaccines. Oncolytic viral therapy. Immune response modifiers. These are several active areas of research as to how Roswell Park’s TII Program is harnessing the power of the immune system as a novel weapon in the battle against cancer.
It is now clear that traditional cancer therapies, such as radiotherapy and chemotherapy, used to fight advanced or metastatic disease, are not enough to further prolong patient survival or enhance their quality of life. Therefore, newer therapeutic interventions are necessary to meet these critical clinical challenges, as well as identifying additional ways the tumor microenvironment and resistance pathways can be modulated to improve therapeutic response.
With a singular focus, the scientific and clinical research activities in Roswell Park’s TII Program have pursued a different path, one that involves developing safe and effective immune-directed therapies, known as immunotherapy. These therapies attack cancer in a very different way – by using a patient's own immune system and cells as a living drug of choice.
Immune cells, known as T lymphocytes, can be collected from the patient and modified in three ways:
- to target specific proteins on the outside of cancer cells to improve immune recognition
- to generate killing potential
- to mobilize additional cells of the immune system to convert “cold” tumors “hot” in order to enhance the density and diversity of the immune response
After cell production, these immune products are returned to the patient as a therapeutic option. Specific ‘chemokine’ cocktails may also be given to patients to draw immune cells to the tumor or additional approaches may include combinations with standard-of-care. Our goal is to generate durable responses in patients that offer reduced side effects, a good quality of life, and improved outcomes.
Program leaders
Program membership highlights
- 30 members
- 11 Departments represented
- 14 Cancers represented
- 13 Clinicians/Clinician Scientists
Our multidisciplinary team of scientists and clinicians have been at the forefront of tumor immunology and immunotherapy research that not only focus on the major cancers in our community or catchment area (lung, breast, colon, and prostate), but also on aggressive, rare, or hard to treat cancers (ovarian, pancreatic, hematologic). Our team works alongside experts in biostatistics and bioinformatics, flow and image cytometry, and vector production, to name a few, so we can develop, test and analyze the effects of these novel immunotherapies, identifying novel biomarkers of efficacy and response, or new therapeutic targets.
Our team collaborates with basic and translational scientists to bring discoveries from the laboratory into safe and feasible pilot trials with our Early Phase Clinical Trials program. We actively train and mentor, in collaboration with our Cancer Research Training and Education Coordination (CRTEC) team, the next generation of early-stage scientists and physician-scientists to ensure a well-trained, dynamic, and diverse group of experts are born to develop even newer ways to manage and eradicate cancer, whether here in our catchment area or beyond.
Moreover, we actively interact with Roswell Park’s Community Outreach and Engagement (COE) team, along with cancer advocates and survivors through the ROCKstar’s program, to focus on the needs of our cancer patients, bring scientific knowledge back to our community, including underserved populations, and partner with them to secure additional funding to establish more innovative and effective treatments. All these efforts are ways we contribute to our vision of:
Global leadership
Driving innovation in tumor immunology research and striving for global recognition in immunotherapy applications.
Empowerment
Mentoring the next generation of immuno-oncology scientists and physician-scientists across the continuum of pre-doctoral to junior faculty levels.
Community impact
Collaborating with COE to align our clinical initiatives with our patient needs & increasing healthcare accessibility.
2023 Program highlights
As of 8/1/23
Funding
Cancer peer-reviewed funding
$13.1M
NCI Funding
$10.2M
Total Funding
Over $18M
Grants
Major Team Science grants
10
MPI R01 grants
10
Catchment area-related grants
16
Publications in last five years
Publications generated
828
High impact (>10 impact factor)
35%
Inter-cancer center collaborations
58%
In the news
Spotlight: CAR T-cell therapy: looking back and looking forward
Recent progress indicates a considerably improved mechanistic understanding of CAR T-cell biology and delivers important insights into why some patients achieve durable remissions and others do not.
Read the latest study by Renier Brentjens, MD, PhD and Marco Davila, MD, PhD
Bench-to-bedside collaboration
Taking advantage of the close collaborations among our scientists, our program relies on the validation of results from in vitro systems to in vivo preclinical or animal models.
The information derived from these in vitro and in vivo studies is then utilized to develop novel immune-based strategies, which may be used alone or combined with other conventional or experimental agents, to address important clinical questions. In turn, the answers generated in the clinical setting raise new questions that necessitate a return to the laboratory for detailed preclinical follow-up and testing.
This bi-directional exchange of information between laboratory and clinical investigators provides the opportunity to apply basic science and the underlying molecular mechanisms to clinically relevant questions.
Some highlighted clinical trial studies are below:
SurVaxM
Trial #: NCT02455557
Principal Investigators: Robert Fenstermaker, MD, and Michael Ciesielski, PhD
SurVaxM was discovered, developed, and clinically translated entirely at Roswell Park. Drs. Fenstermaker and Ciesielski and colleagues have developed therapeutic vaccines against survivin, a non-mutated protein that is overexpressed in glioblastoma multiforme (GBM) and multiple types of other aggressive cancers as a novel anti-cancer agent.
Termed SurVaxM, this vaccine is a first-in-class immunogen targeting survivin. The vaccine acts by inducing antitumor immune responses. A Phase II trial (NCT02455557) of SurVaxM with standard-of-care chemo-radiation in newly diagnosed GBM is now complete and recently published in the Journal of Clinical Oncology.
Of the 63 patients evaluable for outcome, 60 (95.2%) remained progression-free 6 months after diagnosis (pre-specified primary end point). Median progression-free survival was 11.4 months and median overall survival was 25.9 months. Additional clinical trials led by this team involving this cancer vaccine are ongoing, both in GBM (NCT05163080, NCT04013672) and other cancer types, including multiple myeloma (NCT02334865) or neuroendocrine tumors (NCT03879694).
Tivozanib
Trial #: NCT01835223
Principal Investigator: Yasmin Thanavala, PhD
Dr. Thanavala and colleagues have continued their efforts to understand immune dysfunction in patients with advanced hepatocellular carcinoma (HCC).
Their earlier work demonstrated that treatment of HCC patients with sorafenib (a tyrosine kinase inhibitor) reduced the extent of immune suppression caused by regulatory T cells (Tregs), which was associated with increased overall survival.
Her team has extended this work to a newer generation of a more potent kinase inhibitor, termed Tivozanib and evaluated its impact on Tregs, as part of a Phase Ib/II trial (NCT01835223).
Clinical trials in ovarian cancer
Trial #: NCT04919629
Principal Investigator: Emese Zsiros, MD, PhD
This Phase II trial studies the safety of APL-2 (Pegcetacoplan) in combination with Pembrolizumab; or in combination with Pembrolizumab and Bevacizumab; or Bevacizumab treatment alone, in patients with recurrent ovarian cancer that have symptomatic malignant effusions. The study will also evaluate if the combination of these drugs impacts the accumulation of malignant effusions as a measurement of disease control. APL-2 inhibits the complement pathway of the immune system which, in cancer, may be paradoxically pro-tumorigenic. APL-2 is FDA-approved for the treatment of adults with paroxysmal nocturnal hemoglobinuria (PNH), a rare blood disorder, but it has not been evaluated in cancer. Studies in preclinical models and cancer patient biospecimens show that preventing complement activation can improve the antitumor immune response. The clinical trial concept has strong foundational scientific data to support the novel combination of these new drugs together.
Trial #: NCT05231122
Principal Investigator: Emese Zsiros, MD, PhD
This Phase II trial studies the safety and efficacy of CDX-1140 treatment when given in combination with Pembrolizumab and Bevacizumab, compared to the combination of Pembrolizumab and Bevacizumab alone, in patients with recurrent ovarian cancer. CDX-1140 works by stimulating certain immune cells within the tumor and, when combined with other immunotherapy agents, may increase tumor-reactive antibody production, a key element of the antitumor response. CDX-1140 is currently being investigated in other clinical trials with patients who have advanced recurrent tumors and has shown to be well tolerated and can be safely combined with Pembrolizumab.
Trial #: NCT06855706
Principal Investigator: Emese Zsiros, MD, PhD
The purpose of this study is to determine whether an automated, personalized physical activity (PA) plan using wearable technology can increase patient drive and motivation to engage in more robust physical activity, compared to standard physical activity recommendations. This trial will be evaluating the number of ovarian or endometrial cancer patients who reach at least 150 minutes of moderate to vigorous physical activity (MVPA) per week. Participants will wear a ‘Fitbit’ devise, and a continuous glucose monitor for the 6-month study intervention period as readouts for changes in integral health parameters and quality-of-life.
Research highlights
Roswell Park has a long history of discovery in tumor immunology, and we’re unique in that each faculty’s research program has a major focus on cancer and tumor immunology.
Comprehensive tumor-immune profiling reveals mediators of paradoxical immune sensitivity in sarcomatoid renal cell carcinoma
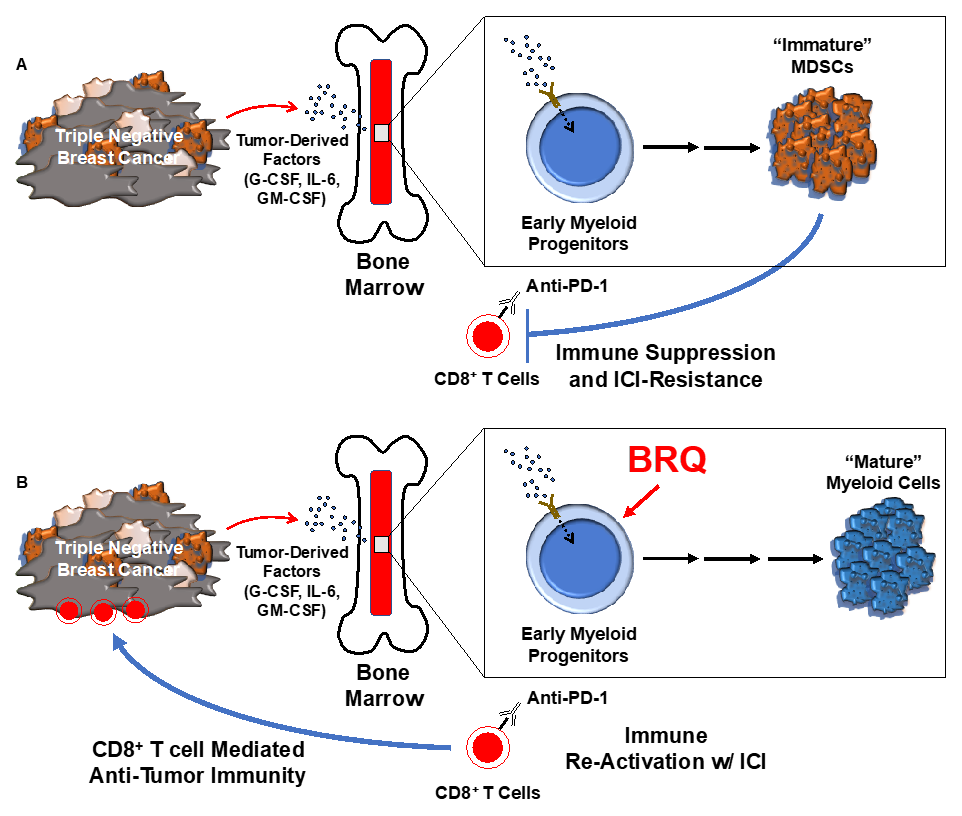
Inhibiting the biogenesis of myeloid-derived suppressor cells enhances immunotherapy efficacy against mammary tumor progression
- Zuo Y, Vohwinkel DJ, Dong B, McDowell JR, Guzman BV, Manickavel Pandian TS, Chattopadhyay S, McGray AJR, Olejniczak SH, Ohm J, Wang J, Long MD, Gomez EC, Purdon TJ, Luo W, Mohammadpour H, Hackett CS, Cherkassky L, Davila M, Abrams SI, Brentjens RJ. IL-36γ armored CAR T cells reprogram neutrophils to induce endogenous antitumor immunity. Cancer Cell. 2025 Dec 11:S1535-6108(25)00500-8. doi: 10.1016/j.ccell.2025.11.007. Epub ahead of print. PMID: 41386225.
- Chiello JL, Shaikh N, Jacobi J, Gaulin N, Santos G, Keck C, Hess SM, Lichty B, Singh PK, Rosario SR, Abrams SI, Zsiros E, Long MD, McGray AJR. BiTE-secreting T cells rationally combine with PD-1 blockade and vaccine boosting to reshape antitumor immunity in ovarian cancer. Mol Ther. 2025 Sep 30:S1525-0016(25)00818-4. doi: 10.1016/j.ymthe.2025.09.047. Epub ahead of print. PMID: 41029902.
- Holling GA, Chavel CA, Sharda AP, Lieberman MM, James CM, Lightman SM, Tong JH, Qiao G, Emmons TR, Giridharan T, Hou S, Intlekofer AM, Higashi RM, Fan TWM, Lane AN, Eng KH, Segal BH, Repasky EA, Lee KP, Olejniczak SH. CD8+ T cell metabolic flexibility elicited by CD28-ARS2 axis-driven alternative splicing of PKM supports antitumor immunity. Cell Mol Immunol. 2024 Mar;21(3):260-274. doi: 10.1038/s41423-024-01124-2. Epub 2024 Jan 18. PMID: 38233562; PMCID: PMC10902291.
- Gandhi S, Opyrchal M, Grimm MJ, Slomba RT, Kokolus KM, Witkiewicz A, Attwood K, Groman A, Williams L, Tarquini ML, Wallace PK, Soh KT, Minderman H, Maguire O, O'Connor TL, Early AP, Levine EG, Kalinski P. Systemic infusion of TLR3-ligand and IFN-α in patients with breast cancer reprograms local tumor microenvironments for selective CTL influx. J Immunother Cancer. 2023 Nov;11(11):e007381. doi: 10.1136/jitc-2023-007381. PMID: 37963636; PMCID: PMC10649898.
- Roy AM, Patel A, Catalfamo K, Attwood K, Khoury T, Yao S, Gandhi S. Racial and Ethnic Disparity in Preoperative Chemosensitivity and Survival in Patients With Early-Stage Breast Cancer. JAMA Netw Open. 2023 Nov 1;6(11):e2344517. doi: 10.1001/jamanetworkopen.2023.44517. PMID: 37991763; PMCID: PMC10665980.
- Jaspers JE, Khan JF, Godfrey WD, Lopez AV, Ciampricotti M, Rudin CM, Brentjens RJ. IL-18-secreting CAR T cells targeting DLL3 are highly effective in small cell lung cancer models. J Clin Invest. 2023 May 1;133(9):e166028. doi: 10.1172/JCI166028. PMID: 36951942; PMCID: PMC10145930.
- Chow J, Khan A, Gaudieri M, Wasik BJ, Conway A, Soh KT, Repasky EA, Schwaab T, Wallace PK, Abrams SI, Singh AK, Muhitch JB. Tumor and immune remodeling following radiotherapy in human renal cell carcinoma. J Immunother Cancer. 2023 Apr;11(4):e006392. doi: 10.1136/jitc-2022-006392. PMID: 37080610; PMCID: PMC10124322.
- Przespolewski A, Goldberg AD, Talati C, Fazal S, Vachhani P, Sanikommu SR, Thota S, Waksal J, Ball B, Famulare C, Stahl M, Baron J, Griffiths EA, Thompson JE, Sweet K, Wang ES. Safety and efficacy of CPX-351 in younger patients (<60 years old) with secondary acute myeloid leukemia. Blood. 2023 Mar 23;141(12):1489-1493. doi: 10.1182/blood.2022016678. PMID: 36493344.
- Zsiros E, Ricciuti J, Gallo S, Argentieri D, Attwood K, Ji W, Hutson A, Visco P, Coffey D, Riebandt G, Mark J, Varghese A, Hess SM, Furlani T, Fabiano A, Hennon M, Yendamuri S, Kauffman EC, Wooten KE, Hicks WL Jr, Young J, Takabe K, Odunsi K, Case AA, Segal BH, Johnson CS, Kuvshinoff B 2nd, Nurkin S, Paragh G, de Leon-Casasola O. Postoperative Restrictive Opioid Protocols and Durable Changes in Opioid Prescribing and Chronic Opioid Use. JAMA Oncol. 2023 Jan 5. doi: 10.1001/jamaoncol.2022.6278. Epub ahead of print. PMID: 36602807.
- Colligan SH, Amitrano AM, Zollo RA, Peresie J, Kramer ED, Morreale B, Barbi J, Singh PK, Yu H, Wang J, Opyrchal M, Sykes DB, Nemeth MJ, Abrams SI. Inhibiting the biogenesis of myeloid-derived suppressor cells enhances immunotherapy efficacy against mammary tumor progression. J Clin Invest. 2022 Dec 1;132(23):e158661. doi: 10.1172/JCI158661. PMID: 36453551; PMCID: PMC9711879.
- Oliver L, Alvarez R, Diaz R, Valdés A, Colligan SH, Nemeth MJ, Twum DYF, Fernández A, Fernández-Medina O, Carlson LM, Yu H, Eng KH, Hensen ML, Rábade-Chediak ML, Fernández LE, Lee KP, Perez L, Muhitch JB, Mesa C, Abrams SI. Mitigating the prevalence and function of myeloid-derived suppressor cells by redirecting myeloid differentiation using a novel immune modulator. J Immunother Cancer. 2022 Sep;10(9):e004710. doi: 10.1136/jitc-2022-004710. PMID: 36150744; PMCID: PMC9511656.
- Mailankody S, Devlin SM, Landa J, Nath K, Diamonte C, Carstens EJ, Russo D, Auclair R, Fitzgerald L, Cadzin B, Wang X, Sikder D, Senechal B, Bermudez VP, Purdon TJ, Hosszu K, McAvoy DP, Farzana T, Mead E, Wilcox JA, Santomasso BD, Shah GL, Shah UA, Korde N, Lesokhin A, Tan CR, Hultcrantz M, Hassoun H, Roshal M, Sen F, Dogan A, Landgren O, Giralt SA, Park JH, Usmani SZ, Rivière I, Brentjens RJ, Smith EL. GPRC5D-Targeted CAR T Cells for Myeloma. N Engl J Med. 2022 Sep 29;387(13):1196-1206. doi: 10.1056/NEJMoa2209900. PMID: 36170501.
- Davila ML, Brentjens RJ. CAR T cell therapy: looking back and looking forward. Nat Cancer. 2022 Dec;3(12):1418-1419. doi: 10.1038/s43018-022-00484-w. PMID: 36539498.
- Ahluwalia MS, Reardon DA, Abad AP, Curry WT, Wong ET, Figel SA, Mechtler LL, Peereboom DM, Hutson AD, Withers HG, Liu S, Belal AN, Qiu J, Mogensen KM, Dharma SS, Dhawan A, Birkemeier MT, Casucci DM, Ciesielski MJ, Fenstermaker RA. Phase IIa Study of SurVaxM Plus Adjuvant Temozolomide for Newly Diagnosed Glioblastoma. J Clin Oncol. 2022 Dec 15:JCO2200996. doi: 10.1200/JCO.22.00996. Epub ahead of print. PMID: 36521103.
- Segal BH, Giridharan T, Suzuki S, Khan ANH, Zsiros E, Emmons TR, Yaffe MB, Gankema AAF, Hoogeboom M, Goetschalckx I, Matlung HL, Kuijpers TW. Neutrophil interactions with T cells, platelets, endothelial cells, and of course tumor cells. Immunol Rev. 2022 Dec 16. doi: 10.1111/imr.13178. Epub ahead of print. PMID: 36527200.
- Alam A, Levanduski E, Denz P, Villavicencio HS, Bhatta M, Alhorebi L, Zhang Y, Gomez EC, Morreale B, Senchanthisai S, Li J, Turowski SG, Sexton S, Sait SJ, Singh PK, Wang J, Maitra A, Kalinski P, DePinho RA, Wang H, Liao W, Abrams SI, Segal BH, Dey P. Fungal mycobiome drives IL-33 secretion and type 2 immunity in pancreatic cancer. Cancer Cell. 2022 Feb 14;40(2):153-167.e11. doi: 10.1016/j.ccell.2022.01.003. Epub 2022 Feb 3. PubMed PMID: 35120601; PubMed Central PMCID: PMC8847236.
- Orr B, Mahdi H, Fang Y, Strange M, Uygun I, Rana M, Zhang L, Suarez Mora A, Pusateri A, Elishaev E, Kang C, Tseng G, Gooding W, Edwards RP, Kalinski P, Vlad AM. Phase I Trial Combining Chemokine-Targeting with Loco-Regional Chemoimmunotherapy for Recurrent, Platinum-Sensitive Ovarian Cancer Shows Induction of CXCR3 Ligands and Markers of Type 1 Immunity. Clin Cancer Res. 2022 Jan 19. doi: 10.1158/1078-0432.CCR-21-3659. Epub ahead of print. PMID: 35046055.
- He X, Zhou S, Huang WC, Seffouh A, Mabrouk MT, Morgan MT, Ortega J, Abrams SI, Lovell JF. A Potent Cancer Vaccine Adjuvant System for Particleization of Short, Synthetic CD8+ T Cell Epitopes. ACS Nano. 2021 MAR 23; 15(3):4357-4371. DOI: 10.1021/acsnano.0c07680. 2021 Feb 19. PubMed PMID: 33606514.
- Tzetzo SL, Abrams SI. Redirecting macrophage function to sustain their "defender" antitumor activity. Cancer Cell. 2021 MAR 13; . DOI: 10.1016/j.ccell.2021.03.002. 2021 Mar 13. PubMed PMID: 33798473.
- Zsiros E, Lynam S, Attwood KM, Wang C, Chilakapati S, Gomez EC, Liu S (GG), Akers S, Lele S (DT), Frederick PJ, Odunsi K. Efficacy and Safety of Pembrolizumab in Combination With Bevacizumab and Oral Metronomic Cyclophosphamide in the Treatment of Recurrent Ovarian Cancer: A Phase 2 Nonrandomized Clinical Trial. JAMA Oncol. 2021 JAN 01; 7(1):78-85. DOI: 10.1001/jamaoncol.2020.5945. PubMed PMID: 33211063; PMCID: PMC7677872.
- Dey P, Kimmelman AC, DePinho RA. Metabolic Codependencies in the Tumor Microenvironment. Cancer Discov. 2021 May;11(5):1067-1081. DOI: 10.1158/2159-8290.CD-20-1211. Epub 2021 Jan 27. PMID: 33504580; PMCID: PMC8102306.
- Gandhi S, Pandey MR, Attwood K, Ji W, Witkiewicz AK, Knudsen ES, Allen C, Tario JD, Wallace PK, Cedeno CD, Levis M, Stack S, Funchain P, Drabick JJ, Bucsek MJ, Puzanov I, Mohammadpour H, Repasky EA, Ernstoff MS. Phase I Clinical Trial of Combination Propranolol and Pembrolizumab in Locally Advanced and Metastatic Melanoma: Safety, Tolerability, and Preliminary Evidence of Antitumor Activity. Clin Cancer Res. 2021 Jan 1;27(1):87-95. doi: 10.1158/1078-0432.CCR-20-2381. Epub 2020 Oct 30. PMID: 33127652; PMCID: PMC7785669.
Education & training
In addition to our research and clinical trials, we are also committed to training the next generation of basic and clinical scientists, who will lead the fight against cancer.
Trainees in Roswell Park’s Tumor Immunology PhD track study closely with program members and participate in all phases of basic and translational research.
Contact us
Tracy Durski
Program Administrator
Phone: 716-845-3256
Email: Tracy.Durski@RoswellPark.org