Cellular adaptation to fuel availability is critical for major cellular decisions, and it requires alterations in metabolic pathways coupled with differential expression of genes to rewire biochemical processes.
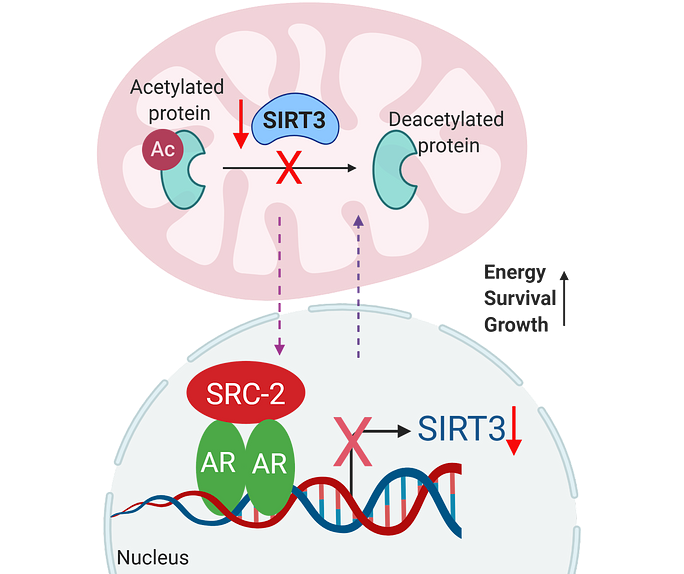
Mitochondria lay at the core of cellular metabolism, whereas nucleus integrates cellular and environmental signals to activate gene transcription. Intriguingly, many metabolic enzymes can directly sense the nutrient supply and stimulate gene transcription to establish an adaptive response.
However, how the mitochondrial enzymes modulate gene transcription in response to bioenergetic stress is poorly understood.
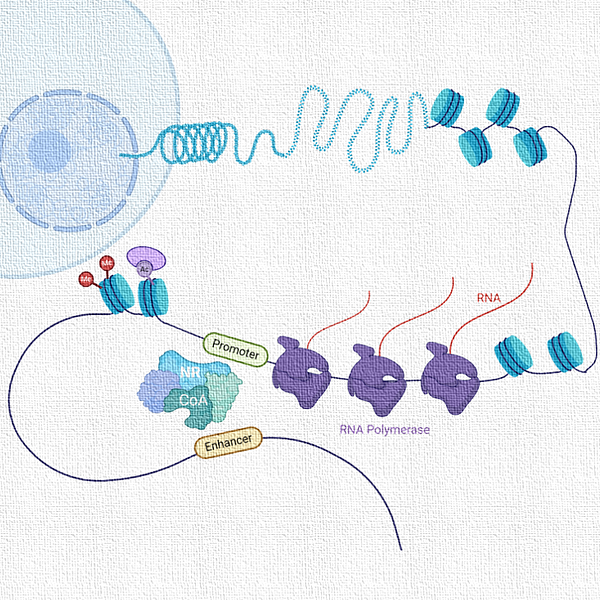
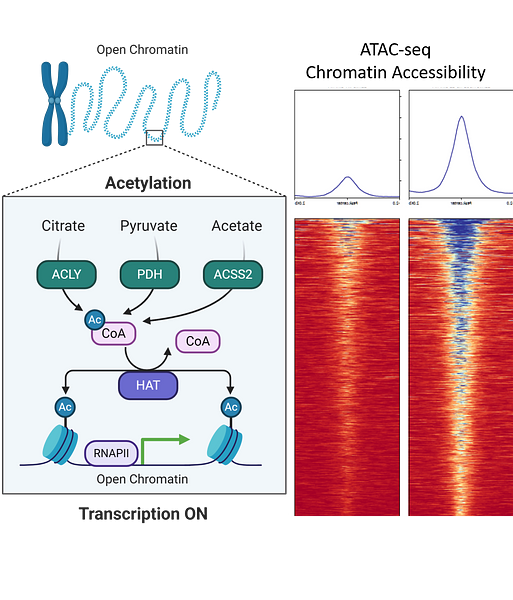
One of the most critical steps before transcription could be initiated is the process of chromatin relaxation which is achieved by acetylation of histone proteins.
Acetylation of the lysine (K) residues in the N-terminal tail of histone reduces its affinity to the DNA, thereby loosening the chromatin and providing greater accessibility to the transcription factors and coregulators for assembly on gene promoters. Thus, acetylation marks on histones dictate gene regulation in an epigenetic manner.
Central to this epigenetic regulation remains the “availability of acetyl-CoA" in the nucleus, which is used by the histone acetyl transferases (HATs) for acetylating histone lysine residues.
This raises an intriguing scientific question, how acetyl-CoA pool is regulated and maintained in the nucleus to sustain gene activation?
Contact the Dasgupta Lab
Email: Subhamoy.Dasgupta@RoswellPark.org
Phone: 716-845-2461 or 716-845-2462
Lab Location: Center for Genetics & Pharmacology (CGP) L3-101- 106
Department of Cell Stress Biology
Roswell Park Comprehensive Cancer Center
Elm and Carlton Streets
Buffalo, NY 14263