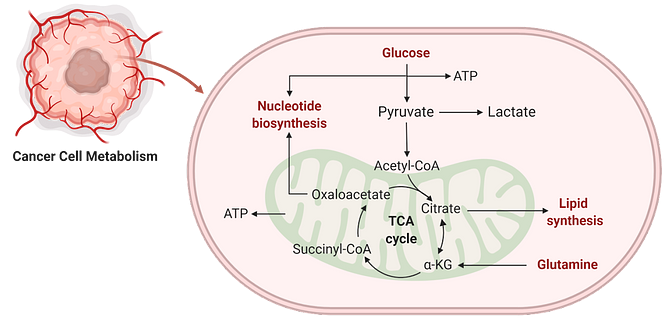
Metabolic adaptation is one of the essential hallmarks of cancer to sustain replication and survival stress. Based on available nutrients, tumor cells alter their metabolic pathways for the biosynthesis of macromolecules and mitochondrial ATP synthesis.
Metabolic reprogramming plays a pivotal role in tumor cell survival during metastatic dissemination, circulation, and colonization in distant organs, thus driving the successful formation of metastatic lesions. Hence, understanding the metabolic checkpoints that drive aggressive metastatic cancer holds promise as an effective therapeutic strategy.
Dysregulation of lipid metabolism is a hallmark of many tumors. The increased demand for de novo lipogenesis is supported by elevated levels of rate-limiting enzymes such as fatty acid synthase (FASN) and stearoyl-CoA desaturase, which are transcriptionally regulated in the nucleus.
Mitochondrial citrate is the precursor metabolite required for lipogenesis, which is exported to the cytosol for conversion into acetyl-CoA and subsequently into malonyl CoA for de novo biosynthesis of fatty acids. But how the nuclear transcriptional regulators communicate with mitochondria to signal sustained synthesis of citrate remains unknown.
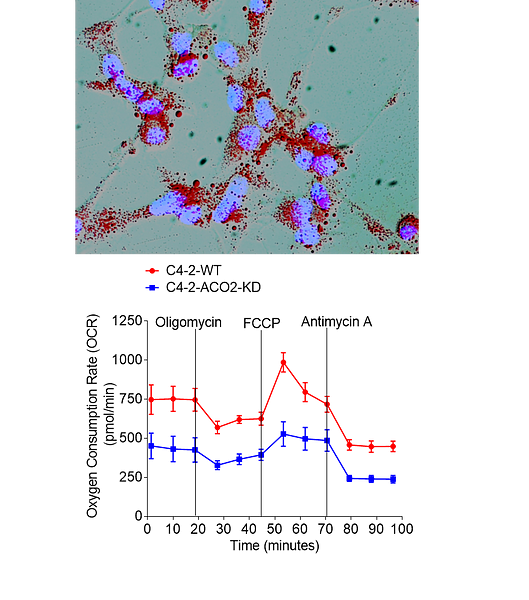
We found mitochondrial aconitase (ACO2) plays a vital role in mitochondrial-nuclear communication, and currently we are investigating underlying mechanisms and enzymatic regulation that promotes lipogenesis in aggressive tumors.
Contact the Dasgupta Lab
Email: Subhamoy.Dasgupta@RoswellPark.org
Phone: 716-845-2461 or 716-845-2462
Lab Location: Center for Genetics & Pharmacology (CGP) L3-101- 106
Department of Cell Stress Biology
Roswell Park Comprehensive Cancer Center
Elm and Carlton Streets
Buffalo, NY 14263