Many women with ovarian cancer initially respond to chemotherapy but eventually relapse because their cancer becomes resistant to standard treatment. This occurs for a variety of reasons, including:
- slow and insufficient immune response
- T lymphocytes (T cells) – the body’s cancer- and infection-fighting cells – becoming “exhausted”
- tumors which recruit cells and other factors that actually prevent the immune system from working properly
- a suppressive tumor environment which makes it difficult for drugs and immune cells to access the cancer cells they are targeting
- a failure by dendritic cells (DC) to present the correct information to T cells to enable them to find and destroy cancer cells.
The two individual research projects (IRP) being conducted as part of the Roswell Park-University of Chicago Ovarian Cancer SPORE aim to make the immune system work better against ovarian cancer.
IRP 1: Reprogramming the immunosuppressive ovarian TME with OV and a CXCR4 antagonist
Co-Leaders: Danuta Kozbor, PhD (Basic); Kunle Odunsi, MD, PhD (Clinical)
Brief summary
The goal of IRP1 is to test in a phase I/II clinical trial an innovative viral therapy administered directly into the site of the tumor, in combination with standard chemotherapy and immunotherapy. The hope is that the viral therapy will reverse the immunosuppressive tumor microenvironment and stimulate better T cell responses, thereby providing a new treatment option for women with chemotherapy-resistant ovarian cancer. Importantly, administering the treatment directly to the site of the tumor is expected to reduce the risk of systemic toxicities.
Detailed summary
The goal of our studies is to generate efficacious tumor-specific T cell responses in patients with chemotherapy-resistant high-grade serous ovarian cancer (HGSOC). Key stumbling blocks underpinning immunotherapy resistance against HGSOC include:
- insufficient expansion of tumor antigen-specific T cells
- recruitment of T regulatory and myeloid-derived suppressor cells via tumor CXCL12 production
- exhaustion of tumor-infiltrating T lymphocytes (TILs) associated with upregulation of PD1
- low tumor immunogenicity due to reduced mutation load and IFNβ production
- insufficient recruitment of intratumoral dendritic cells
- disorganized tumor vasculature incapable of supporting TIL trafficking
Herein, we propose to target these immune resistance mechanisms using an innovative, clinically translatable viral oncotherapy combination for effective direct oncolysis and reprogramming antitumor immunity.
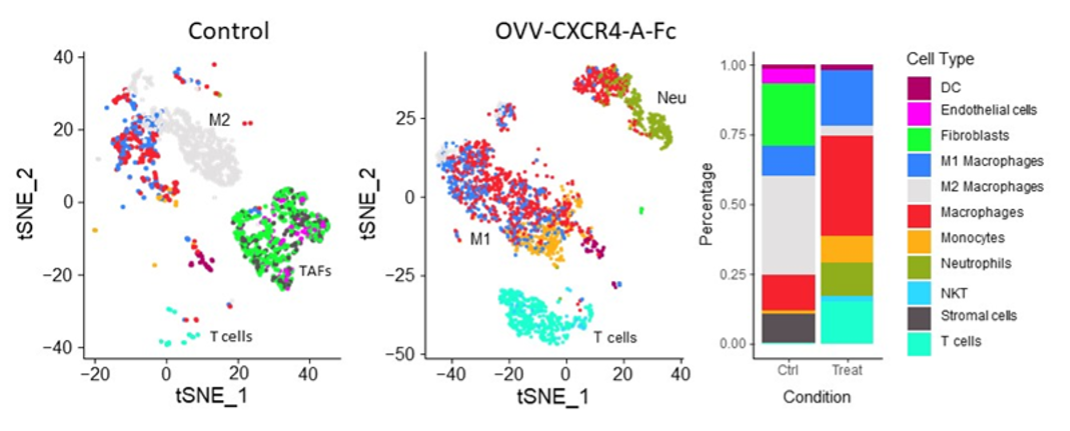
The scientific premise of this project stems from our discovery that blockade of the CXCL12 chemokine/CXCR4 receptor axis in the chemotherapy-resistant ovarian cancer by intraperitoneal delivery of an oncolytic vaccinia virus expressing a CXCR4 antagonist (OVV-CXCR4-A-Fc) in combination with pegylated liposomal doxorubicin (PLD) yields a significant therapeutic impact by reversing the immunosuppressive TME and stimulating rigorous T cell responses (depicted in the graphic abstract below). Our proposed, mechanistic studies conducted in a phase I/II clinical trial will test the hypothesis that in patients receiving liposomal doxorubicin for platinum-resistant/refractory HGSOC, in vivo tumor destruction by OVV-CXCR4-A-Fc will provide clinical benefit by:
- abrogating tumor immune suppression
- promoting the accumulation of TILs
- when combined with PDL1 blockade, limiting T cell exhaustion
The approach is to first determine whether intraperitoneal OVV-CXCR4-A-Fc safely transforms the ovarian TME from tolerogenic to immunogenic in a first-in-human clinical trial in patients with platinum-resistant/refractory HGSOC. Second, we will establish whether OVV-CXCR4-A-Fc combined with anti-PDL1 antibody treatment is safe and clinically efficacious. Third, we will test whether the combinatorial regimen generates functionally distinct tumor-specific CD8+ and CD4+ effector/memory cells. Finally, we plan to uncover the molecular mechanisms by which OVV-CXCR4-A-Fc overcomes the ovarian tumor “vascular checkpoint” to enhance T cell migration and trafficking.
IRP 4: Enhancing Personalized Care in Ovarian Cancer Through Prospective Exercise Interventions and AI-Driven Data Integration
Co-Leaders: Kirsten Moysich, PhD (Overall); Emese Zsiros, MD, PhD, FACOG (Clinical); Rikki Cannioto, PhD, EdD, MS (Applied)
Brief summary
While exercise has been shown to improve fat composition and lean muscle mass, two strong predictors of survival outcomes in ovarian cancer (OC) patients, as well as enhance anti-tumor responses and bacterial microbiome richness in the gut, extensive interventional studies that leverage this understanding to improve OC outcomes are lacking. We propose an automated, artificial intelligence (AI)-driven prospective, randomized clinical trial to track lifestyle factors using Fitbit and continuous glucose monitoring, social, and psychological factors indicative of wellness and metabolic health, combined with electronic health records and advanced AI data integration to test the benefits of an exercise intervention and identify predictors of improved outcomes.
Detailed summary
Exercise has been shown to improve outcomes in ovarian cancer (OC) patients by improving muscle mass, fat composition, gut microbiome diversity, and anti-cancer responses. However, understanding how to effectively use exercise or physical activity (PA) and other behavioral approaches that may improve OC outcomes is lacking. Previous studies evaluating PA in cancer patients relied on self-reported questionnaires, leading to reporting biases and the need for reliable tools. We have previously demonstrated the feasibility of using Fitbit tracking devices in 27 OC patients, successfully integrating their data into the EHR. Our prior work has shown that these devices can reliably track PA, heart rate variability, and sleep patterns, providing valuable insights into patients' daily routines. In addition, self-reported PA and sleep in OC showed little correlation with objective real-time tracking quality of life (QoL) factors. This emphasizes the importance of collecting these data and providing behavioral modifications to improve sleep and reduce a sedentary lifestyle. We aim to build on these studies by collecting additional data to create artificial intelligence (AI)-driven predictive models that enhance our understanding of patient outcomes and guide personalized treatment strategies, as well as offer a PA intervention that may impact various factors such as muscle mass, fat composition, physical functioning and QoL.
We propose a prospective, randomized clinical trial for 60 newly diagnosed or recurrent OC patients with evidence of disease. These patients will be randomized to a PA intervention arm where patients will be given artificial intelligence (AI)-generated prompts to exercise >150 minutes/week or a control arm with initial PA counseling only (Specific Aim 1). Metabolic health will also be measured using a continuous glucose monitor (CGM), and social and psychological factors indicative of wellness, combined with electronic health records and advanced AI data integration, will test the benefits of a PA intervention and identify predictors of improved outcomes. Our AI-validated algorithm that correlates with OC patient overall survival and segments CT scans to calculate body fat and muscle compositions will be utilized. These analyzed scans, along with additional QoL and resiliency questionnaires, will also be incorporated (Specific Aim 2). Our hypothesis is that increased PA will lead to meaningful changes in body composition, improved mobility, and QoL, ultimately leading to better survival outcomes. We also hypothesize that data collected from CGMs will provide valuable information about a patient's metabolic health (daily glucose trends, variability, time in range). Integrating these diverse data sources using AI-driven methodology, including activity trackers, CGMs, advanced imaging analysis, and social and psychological factors, will allow us to create robust predictive models, including developing digital twin models, to enhance our understanding of patient outcomes and guide personalized treatment strategies (Specific Aim 3).
Contact us