Leveraging polyamine metabolism in prostate cancer toward novel therapeutic approaches
A major clinical issue in prostate cancer treatment is that despite androgen deprivation being an excellent therapy to treat even advanced metastatic disease, patients recur with more aggressive disease that is no longer responsive to androgen deprivation.
Prostate cancer cells use a myriad of adaptations to get around the fact that therapy has profoundly limited circulating levels of testosterone. We wanted to find a novel approach independent of targeting androgens and focused on a prostate-unique aspect of metabolism.
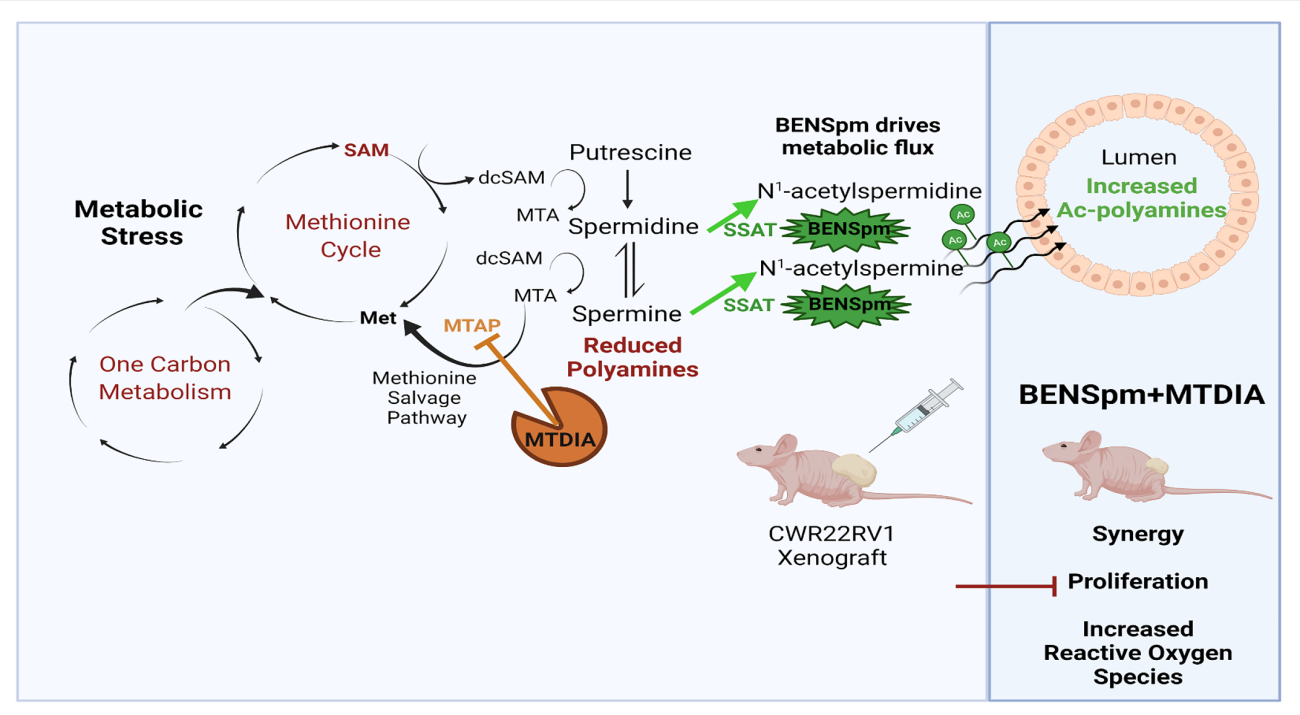
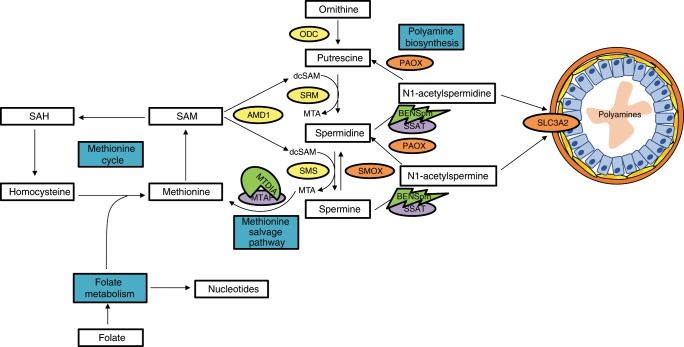
The prostate makes extraordinary levels of polyamines that get secreted into the lumen, where they are a critical component of prostatic fluid with a key role in fertilization. The high flux through the polyamine biosynthetic pathway, due to the secretion of polyamines, is unique to the prostate and provides a potential therapeutic window to target prostate cells. Prostate cancer maintains this high flux through polyamine biosynthesis.
We found we could increase the stress on the system by increasing the secretion of polyamines if we used the drug BENSpm. This, in turn, increases metabolic stress on the connected methionine cycle and one-carbon metabolism. The prostate cells use the methionine salvage pathway to help relieve this stress because it allows for recovery of one-carbon units that would otherwise be lost to polyamine biosynthesis.
We also found we could block the methionine salvage pathway with the drug MTDIA and that this blocked the cells’ ability to relieve the stress of high flux through polyamine biosynthesis. The dual approach, increasing stress while blocking the ability to mitigate the stress, was synergistic in blocking prostate cancer proliferation in the androgen deprivation setting. Ultimately, the hope is this combined approach will prevent recurrence during traditional androgen deprivation.
We are continuing this line of research by trying to find additional ways to take advantage of the metabolic stress induced because of the unique polyamine metabolism found in the prostate and prostate cancer. Metabolomic analyses in mice with prostate cancer xenografts have demonstrated that the vast majority of metabolite level changes induced by the combination therapy are focused on the cancer with very limited effects in normal tissues. This validates the idea of a therapeutic window to take advantage of.
Connect with the Smiraglia Lab
Department of Cell Stress Biology
Center for Genetics & Pharmacology (CGP) L3-107-110
Roswell Park Comprehensive Cancer Center
Elm and Carlton Streets
Buffalo, NY 14263