Understanding the role of NCOR2 in regulating cell identity in castration recurrent prostate cancer
A major clinical issue in prostate cancer treatment is that despite androgen deprivation being an excellent therapy to treat even advanced metastatic disease, patients recur with more aggressive disease, no longer responsive to androgen deprivation.
Prostate cancer cells use a myriad of adaptations to get around the fact that therapy has profoundly limited circulating levels of testosterone. As we get better at targeting adaptations to changes in things like androgen production or dysregulated androgen receptor function, we are finding a new method of adaptation.
Prostate cancer cells manage to change their identity such that they no longer need androgen signaling. One such adaptation is for the cells to take on a neuroendocrine phenotype to create a highly aggressive neuroendocrine prostate cancer.
NCOR2 is a nuclear receptor co-repressor that helps to regulate the epigenome by recruiting histone deacetylases to regions where NCOR2 is recruited. This results in a closed chromatin structure that is not conducive to transcription.
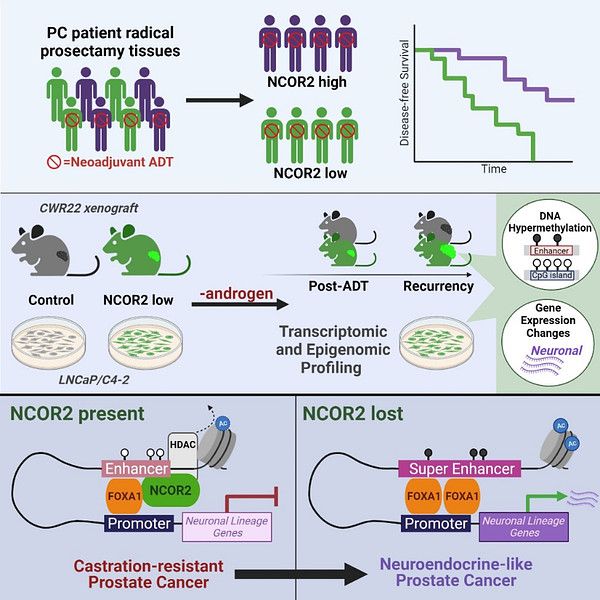
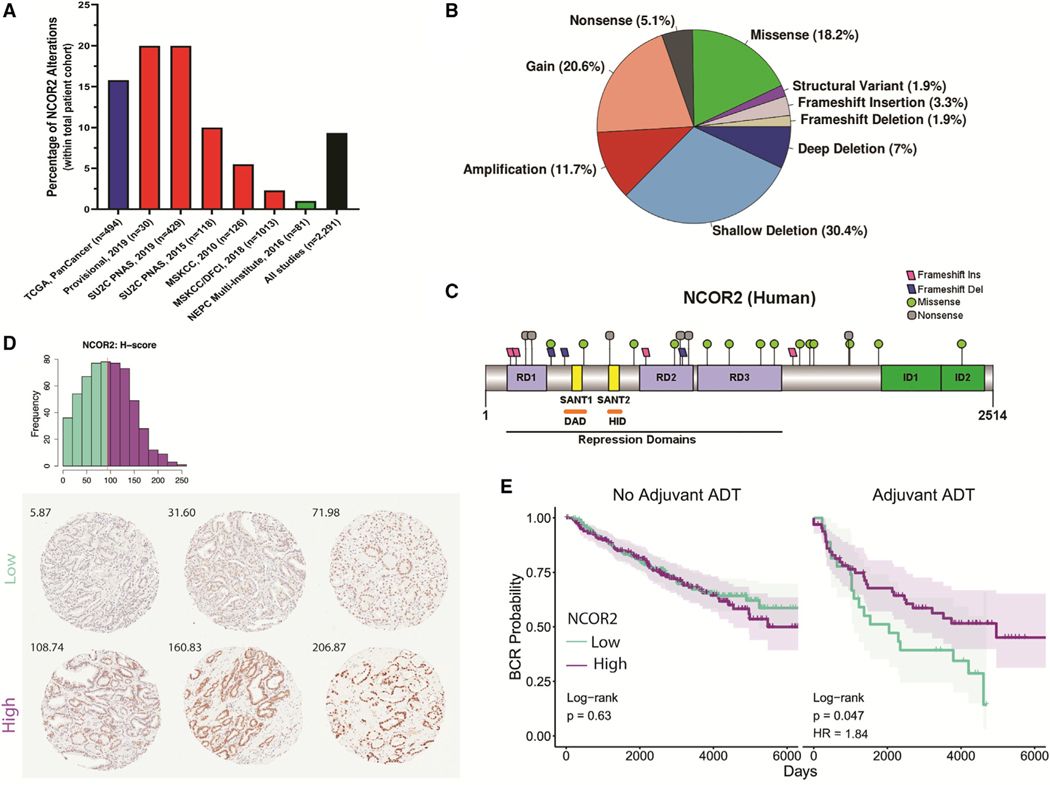
We found that loss of function mutations in NCOR2 were relatively frequent in castration recurrent prostate cancer. Furthermore, in a cohort of men who received neoadjuvant androgen deprivation therapy, we found reduced levels of NCOR2 correlated with faster biochemical recurrence after prostatectomy.
We asked how loss of function associated with time to recurrence and the nature of the recurrence. Using a patient-derived xenograft model (CWR22) of the transition between androgen-dependent disease and recurrent disease where we could reduce NCOR2 expression (or not), we found that reduced NCOR2 accelerated recurrence.
“In all of our analyses, we found that loss of NCOR2 not only accelerated prostate cancer recurrence, but also induced a shift in cellular identity to neuroendocrine prostate cancer, which is a more lethal form of the disease.”
See the scienceRead the press releaseWe also found greatly increased DNA methylation and enrichment for aberrant DNA methylation at super-enhancers that control neuronal lineage-determining genes. Super enhancers are large enhancer hubs that often regulate lineage-determining genes and the role of NCOR2 and DNA methylation in regulating them is unknown.
We are continuing this line of research by asking how loss of NCOR2 affects the structure super-enhancer and how changes in DNA methylation at such super-enhancers might affect their function.
Connect with the Smiraglia Lab
Department of Cell Stress Biology
Center for Genetics & Pharmacology (CGP) L3-107-110
Roswell Park Comprehensive Cancer Center
Elm and Carlton Streets
Buffalo, NY 14263