Advanced Image-Based Treatment Planning
A first-in-human clinical trial demonstrated that image-guided interstitial photodynamic therapy (I-PDT) of refractory extrabronchial malignant central airway obstruction is safe and potentially effective.
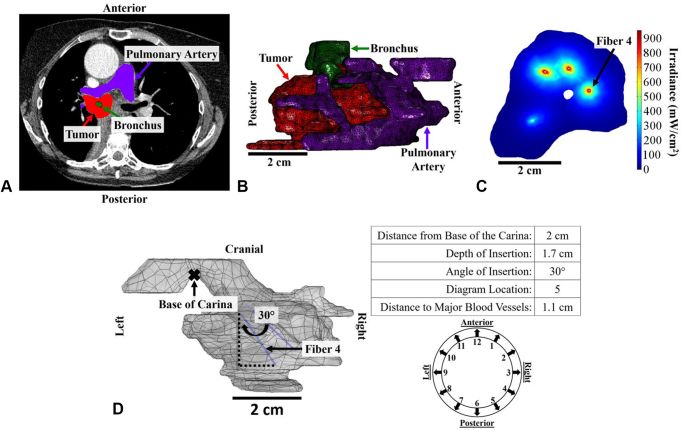
The image below, published in JTO Clinical and Research Reports, shows the Shafirstein Lab’s treatment plan for I-PDT of inoperable non–small cell lung cancer (NSCLC) with an airway obstruction.
- A – A CT scan with outlines of the tumor and pulmonary artery.
- B – Digital mesh demarcating the tumor (red), pulmonary artery (purple) and airway (green).
- C – Cross section of the irradiance distribution from the light diffuser fibers. Range of irradiance, 0.4–955 mW/cm2
- D – Treatment plan for placement of fiber 4. Fiber 4 was placed 2.0 cm from the base of the carina and inserted into the tumor at a 30° angle off the base of the carina and at a depth of 1.7 cm into the target tumor tissue. Fiber 4 was placed at the 5 o’clock position in relation to 12 o’clock as being directly anterior.
This clinical trial is now in Phase II, supported by a National Cancer Institute award through collaboration with Simphotek, Inc.
The integrating image-guided dosimetry also is being used to research “Interstitial Chemophototherapy with Light-Activated Nanoparticulate Doxorubicin” of large hepatocellular carcinoma in collaboration with PoP Biotechnologies, Inc., supported through awards from the NIH/NCI Small Business Technology Transfer (STTR) seed fund.
More active i-PDT clinical trials
NCT03735095
Endobronchial Ultrasound Transbronchial Needle Guided Interstitial Photodynamic Therapy for Palliation of Locally Advanced Lung Cancer and Advanced Cancers Obstructing the Airway - Phase 1/2 Study
View trialNCT03727061
A Randomized, Multi-Center Phase 2 Trial with a Phase 1 Safety Run-in: Porfimer Sodium Mediated Interstitial Photodynamic Therapy and Standard of Care (SoC) Therapy versus SoC Therapy alone for the Treatment of Patients with Locally Advanced or Recurrent Head and Neck Cancer
View trialNCT03678350
Light Dosimetry for Intraoperative Photodynamic Therapy with Porfimer Sodium (Photofrin®) in Patients with Malignant Mesothelioma, Non-Small Cell Lung Cancer, or Other Malignancies with Pleural Disease - Phase 1 Study
View trialNCT04836429
Utilizing Photodynamic Therapy to Amplify the Response to Immunotherapy in Patients with Non-Small Cell Lung Cancer with Pleural Disease - Phase 1 Study
View trialContact the Shafirstein Lab
Email: Gal.Shafirstein@RoswellPark.org
Phone: 716-845-4025
Department of Cell Stress Biology
Roswell Park Comprehensive Cancer Center
Elm and Carlton Streets
Buffalo, NY 14263