Myeloid-derived suppressor cell ‘differentiation therapy’
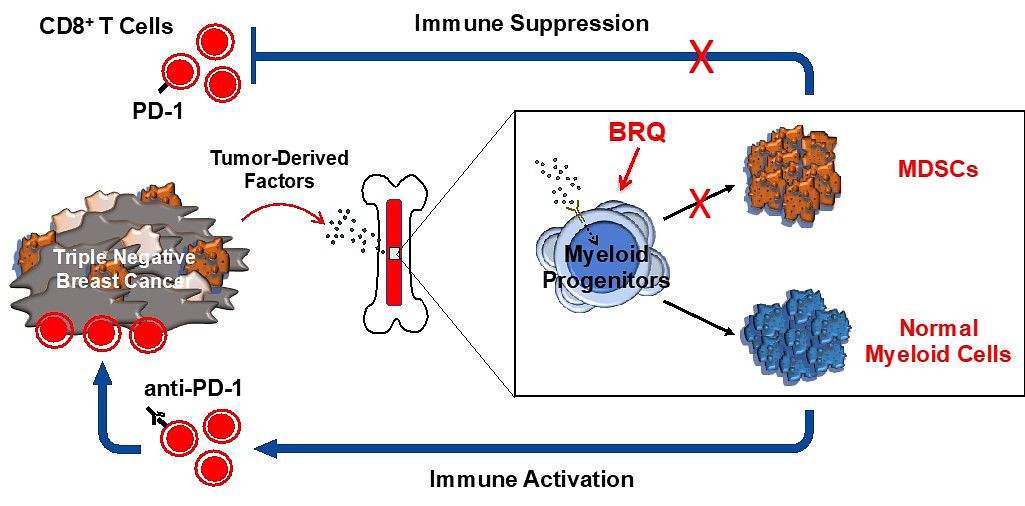
Prominent components of the tumor microenvironment (TME) include the accumulation of myeloid-derived suppressor cells (MDSCs). These cells travel from the bone marrow to the TME where they then inhibit the proliferation or effector functions of CD8+ cytotoxic T cells, hampering their response to immunotherapy, including immune checkpoint inhibitors (ICIs).
To overcome this roadblock, our laboratory, in collaboration with the Nemeth Lab, has developed a novel approach to target MDSC biogenesis in the bone marrow to bolster ICI activity, or perhaps other forms of immunotherapy in preclinical models of triple negative breast cancer (TNBC).
We identified a metabolic susceptibility in MDSCs and target that vulnerability using dihydroorotate dehydrogenase (DHODH) inhibitors. DHODH inhibitors block de novo pyrimidine metabolism and are being used in clinical trials as a therapy to ameliorate acute myeloid leukemia (AML) by enforcing the maturation of the leukemic myeloid progenitors in the bone marrow.
Given similarities in the bone marrow origin of both AML and MDSCs, our laboratories have shown that targeting MDSC biogenesis using a specific and potent DHODH inhibitor known as brequinar (BRQ), facilitates MDSC maturation and dampens their immune suppressive phenotype, all resulting in enhanced CD8+ T cell activity in response to ICI therapy against both primary and metastatic disease in otherwise ICI-resistant preclinical models of TNBC.
Altogether, these data indicate that DHODH blockade in vivo abrogates the pro-tumorigenic behavior of MDSCs, suggesting a novel approach to ‘reprogram’ MDSCs for use in combination immunotherapy protocols where MDSCs are thought to play integral roles in tumor progression and as barriers to treatment outcome, such as in patients with TNBC.
Ongoing studies in our laboratories are investigating mechanisms underlying MDSC mitigation in response to these inhibitors with implications to other myeloid elements within the TME, such as macrophages or dendritic cells, other cell types important for optimal antitumor immunity.
It is our long-term goal that our research will improve knowledge of the host-tumor interaction and suggest ways for this new knowledge to be translated to novel therapeutic opportunities that could reduce the burden of human cancer.
Connect with the Abrams Lab
Department of Immunology
Roswell Park Comprehensive Cancer Center
Elm and Carlton Streets
Buffalo, NY 14263