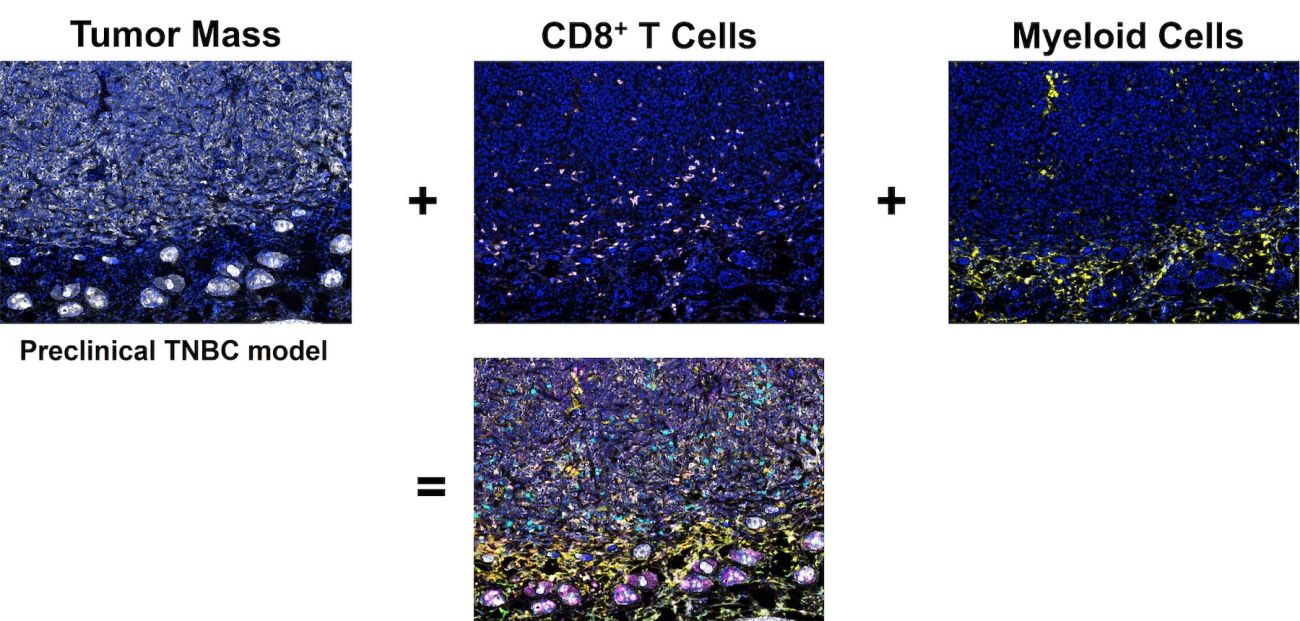
Complexities of the immune-tumor microenvironment
Immune suppression is a dominant form of tumor escape and culminates from a cancer-induced process that antagonizes productive antitumor immune responses, very much like a tug-of-war. Major cellular components that drive immune suppression include the myeloid arm of the innate immune system, namely myeloid-derived suppressor cells (MDSCs) and macrophages.
Together, both populations are an abundant component of the non-cancerous content of solid malignancies, known as the tumor microenvironment (TME).
Within the TME, such myeloid populations are highjacked by the growing malignancy and exhibit multiple immune system-dependent and -independent pro-tumorigenic mechanisms that:
- Fuel cancer cell proliferation
- Remodel the extracellular matrix
- Promote cancer cell invasion and angiogenesis
- Inhibit innate (Natural Killer (NK) cell) and adaptive (CD4+ and CD8+ T cell) immune responses
- Interfere with response to various therapies, including radiotherapy, chemotherapy, and immunotherapy
Therefore, restoring ‘myeloid fitness’ is likely to augment immune surveillance and therapeutic efficacy across these diverse traditional or experimental regimens. In both MDSC and macrophage biology, our laboratory has focused on how these populations develop or acquire their pro-tumorigenic activities. Investigations over the past several years have yielded novel insights into these incompletely understood mechanisms.
Our work uncovered previously unrecognized roles of interferon regulatory factor-8 (IRF8), a myeloid-dependent transcription factor, in both MDSC- and macrophage-tumor biology.
In the case of MDSCs, we discovered that IRF8 expression plays a crucial role affecting their development from the bone marrow, their birthplace. We found that IRF8 functions as an integral negative regulator of MDSC ‘biogenesis’ (or production) in preclinical models of mammary carcinoma, a tumor type that elicits robust MDSC responses.
Mechanistically, we demonstrated that such tumor types secrete chronically high levels of myeloid growth factors, like granulocyte colony-stimulating factor (G-CSF), that drive MDSC biogenesis by downregulating IRF8 expression via STAT3 (signal transducer and transcription activator-3)-dependent pathways.
In the case of macrophages, we observed that tumor-derived G-CSF similarly downregulates IRF8 expression which, in turn, renders these cells pro-metastatic though pathways yet to be fully defined. Interestingly, this outcome was quite prominent in ‘tissue-resident’ macrophage populations of the lung, a common site for metastatic mammary cancer and other solid cancer types.
Thus, our work unveiled a new biology of IRF8 in the regulation of both MDSC and macrophage responses in cancer, one acting at a developmental level and the other acting at a functional level. Projects in the laboratory are building on these concepts and their translational implications to therapeutic applications.
One key project exploits the use of this new knowledge of MDSC biogenesis to improving immunotherapeutic platforms in triple-negative breast cancer (TNBC), a highly aggressive subtype of breast cancer with limited treatment options.
Connect with the Abrams Lab
Department of Immunology
Roswell Park Comprehensive Cancer Center
Elm and Carlton Streets
Buffalo, NY 14263