At the forefront of cancer therapy development and treatment design
How can we use new treatments to target cancer cells and prevent therapy resistance?
Genomics and genetics. Molecular and cellular biology. Biochemistry. Imaging. It’s all part of learning how new drugs — and new combinations of drugs — can treat and prevent cancer.
From discovery and development to translation and clinical trials, the Developmental Therapeutics Program at Roswell Park is making breakthrough discoveries in new cancer therapies.
Our uniquely collaborative team includes scientists and physicians from 10 oncology specialties, working together to unravel the complexities of cancer-signaling drivers, hormone-driven cancers, and resistance mechanisms.
Publications in CY 2022
200
CY 2022 funding (Direct)
$15.4M
Program leaders
Dr. Tang, Chair of Pharmacology & Therapeutics and an expert on cancer stem cells and prostate cancer, primarily focuses on basic science-driven therapy development efforts in the program.
Dr. Dy, a medical oncology specialist on thoracic oncology, leads many clinical trials especially in lung cancer. As an NCI-funded physician scientist, Dr. Dy has also been collaborating with basic scientists here at Roswell Park and University at Buffalo to develop exosome-based and other liquid biopsy biomarkers for early cancer detection and for assessing treatment efficacy and resistance.
Together, they work closely to develop and implement the strategic plan, execute research activities including seminars, retreats, and pilot project funding, and prioritize and facilitate therapeutic development within the Developmental Therapeutics Program and across Roswell Park.
Program goals & aims
The primary goal of Developmental Therapeutics research at Roswell Park is to develop and translate novel anti-cancer therapeutics and therapeutic regimens.
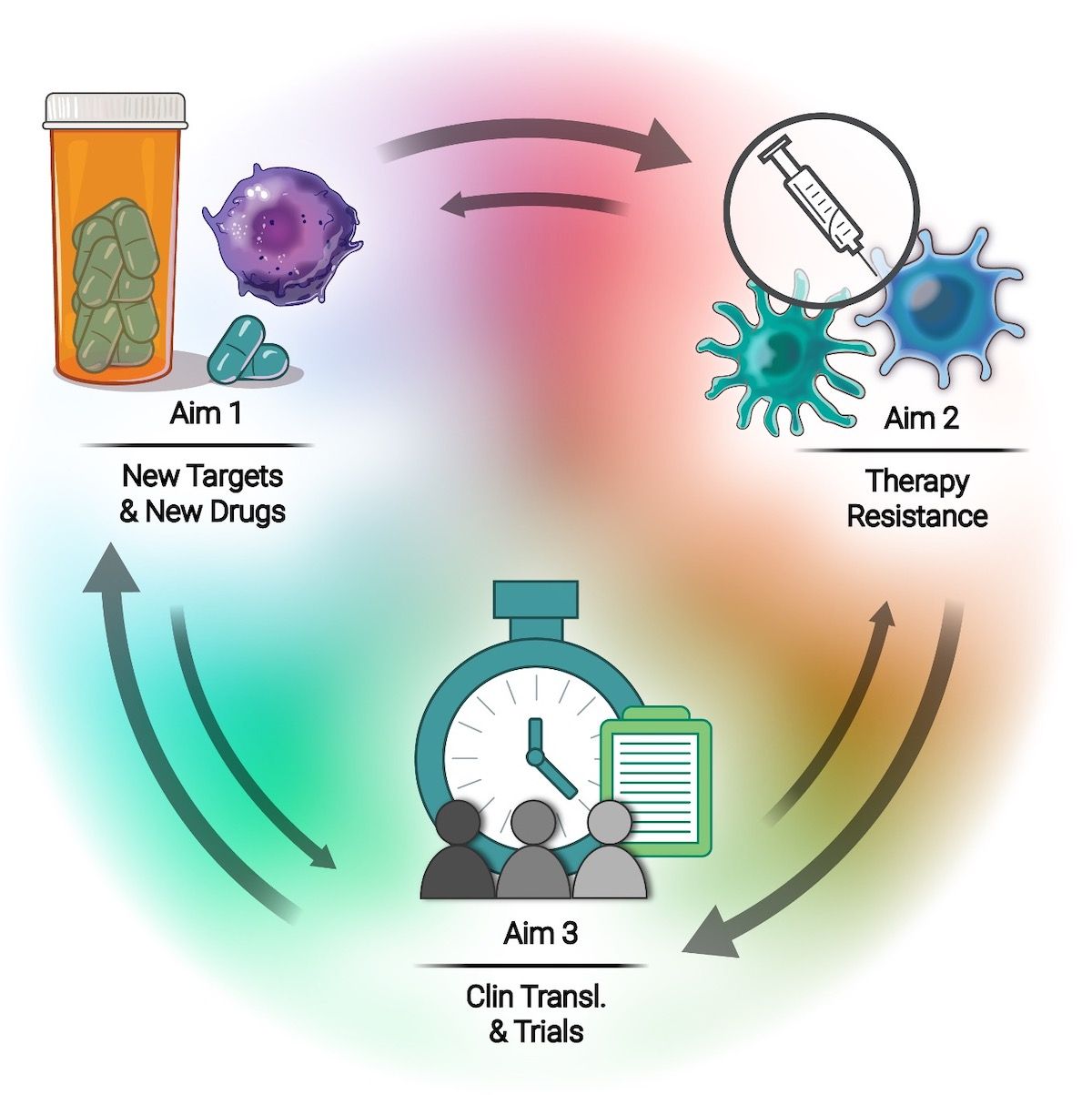
Our highly collaborative, interdisciplinary team focuses on three specific aims:
Aim 1: Uncover new cancer vulnerabilities & develop novel drugs and combinations
- Interrogate deregulated cancer transcriptomes
- Define novel genetic & epigenetic drivers
- Target cancer proteostasis & immunosuppressive TME
Aim 2: Understand & overcome therapy resistance
- Develop novel strategies for targeted therapies and ICBs
- Exploit DDR abnormalities to sensitize to PARPi, ICB and others
- Employs systems biology approaches for novel resistance targets
Aim 3: Facilitate clinical translation & clinical trials
- Facilitate clinical translation of home-grown drugs
- Translate novel program-developed combinations
- Conduct innovative clinical trials
Early Phase Clinical Trials
Through our Center for Early Phase Clinical Trials (CEPCT), we bring scientific findings from the Developmental Therapeutics team and other CCSG programs into the clinic.
The CEPCT is Roswell Park’s resource for:
- Laboratory-based drug discoveries
- Preclinical efficacy studies
- PK/PD modeling and pharmacometric studies
- IND-enabling toxicology
- IND submission support
- Clinical trial conduct and management
This team coordinates and implements complex phase I/II trials from other CCSG programs that have one or more of the following characteristics:
- Disease site agnostic
- “Basket trials” involving multiple different tumor types
- Trials involving adoptive immune cell therapy (excluding hematopoietic stem cell transplantation) due to the complexity of multidisciplinary planning and management
- Trials with unusual complexity in implementation, administration or regulation (e.g., the trials with Cuban vaccines and immunomodulatory agents).
In the past three years, CEPCT has translated recent preclinical findings into 18 ongoing clinical trials by Developmental Therapeutics Program members (7 in Phase I) and 11 trials in collaboration with other CCSG programs (4 in Phase I).
Research highlights
Aggressive Prostate Cancer Marked by Abnormal RNA Splicing, Study Reveals
The team set out to learn how prostate cancer cells differ from normal, healthy prostate cells, in particular how those cancerous cells transcribe their genes. The researchers focused on alternative splicing, the process by which a DNA sequence is transcribed into RNA forms in an abnormal manner compared to healthy cells, resulting in tumors.
Use of Pembrolizumab Provided Long-Term Benefits in Patients with Metastatic Melanoma, 10-Year Look Shows
This retrospective analysis of three randomized clinical trials (KEYNOTE-001, KEYNOTE-002 and KEYNOTE-006) involved 1,558 patients with advanced melanoma and known BRAF tumor status (BRAF wild-type or BRAF V600E/K-mutant melanoma) who had all been treated with pembrolizumab, some of whom had undergone prior treatment with BRAF inhibitors with or without MEK inhibitors.
Education & training
We’re committed to training the next generation of basic and clinician scientists to develop expertise in all phases of drug development. Trainees in Roswell Park’s Experimental Therapeutics PhD track study closely with Developmental Therapeutics program members and participate in all phases of basic and translational research.
Contact us
Sue Bechtel
Program Administrator
Phone: 716-845-5853
Email: Susan.Bechtel@RoswellPark.org