ErbB2 is an important oncogene and anti-tumor target. ErbB2 gene amplification occurs in 20-30% of human breast cancer and is significantly correlated with protein expression in the cancer tissues. ErbB2 amplification or overexpression also occurs in other cancers, e.g., gastric cancer and ovarian cancer. ErbB2 gene amplification or protein overexpression is a strong predictor of poor disease prognosis. ErbB2-targeted therapies, particularly humanized monoclonal antibody trastuzumab (Herceptin) in combination with chemotherapy, shows considerable clinical efficacy. However, approximately 50% of patients show primary or secondary resistance to Trastuzumab. Additionally, trastuzumab must be produced in mammalian cells, making it very cost prohibitive. Ado-trastuzumab emtansine (Kadcyla), a conjugated version of trastuzumab to a microtubule inhibitor, was approved last year for patients with metastatic ErbB2-positive breast cancer, who are resistant to trastuzumab. However, Kadcyla is currently even more expensive than trastuzumab itself, while only showing moderate efficacy. There is an urgent, unmet need for an anti- ErbB2 agent.
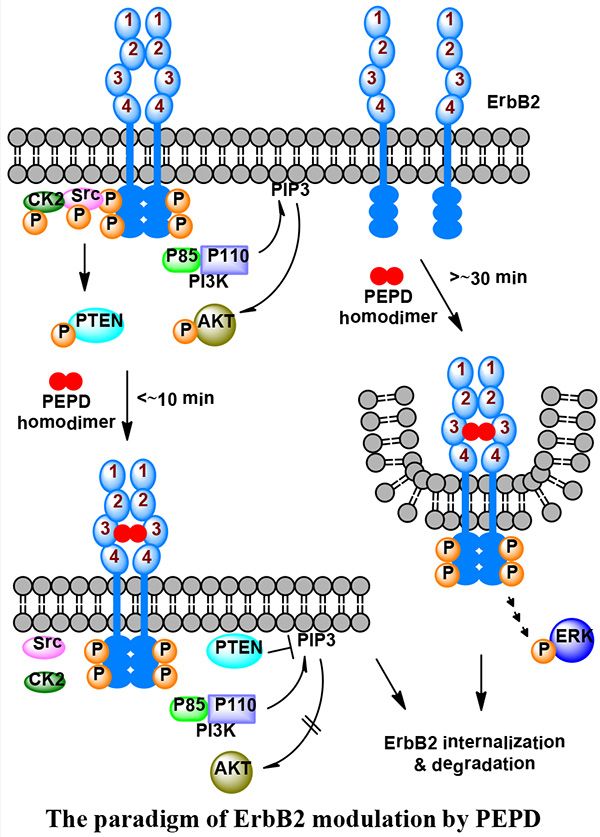
In cancer tissues in vivo, trastuzumab neither downregulates ErbB2 expression nor inhibits ErbB2 tyrosine phosphorylation. Rather, its Fc domain is essential for tumor inhibition by engaging Fc receptors on immune effector cells. We have discovered human prolidase (PEPD) to be an ErbB2 ligand and it strongly targets ErbB2-driven tumors in vivo in animals. However, PEPD does not have an Fc domain. PEPD is an ErbB2 ligand and binds to subdomain 3 of the ErbB2 extracellular domain (ECD). PEPD binding to ErbB2 causes rapid shutdown of ErbB2 oncogenic signaling and also profound depletion of ErbB2 protein via internalization and degradation. Moreover, PEPD also silences other ErbB family members ErbB1 and ErbB3 in tumor tissues via disruption of their heterodimerization with ErbB2 as well as down regulation of ErbB1.
Because the antitumor mechanism of PEPD differs from the antitumor mechanism of trastuzumab in vivo, PEPD is not only a novel anti-ErbB2 agent, but also it may complement trastuzumab and help to overcome resistance to trastuzumab in patients. Moreover, this product can be produced in bacteria using recombinant technology vs. trastuzumab which must be produced in mammalian cells (due to the Fc domain needing glycosylation), thus likely making PEPD a lower cost agent in comparison. Enzymatically inactive mutant of PEPD is even more efficacious than the wild type PEPD in targeting/inhibiting ErbB2 tumors in vivo. Enzymatically inactive PEPD mutant may also allow ErbB2- targeting in cancer in patients without interfering with the normal function of endogenous PEPD.