Organ-specific toxicity is associated with clinically active chemotherapeutic agents and represents challenging health problems. Alopecia is a toxicity effect of chemotherapy-treated patients with breast cancer. Cyclophosphamide, an important chemotherapeutic agent in the treatment of breast cancer, is associated with alopecia and bladder toxicity; as the dose-limiting toxicity affects 20-30% of patients. In patients treated with cisplatin, nephrotoxicity is the dose-limiting toxicity of this drug affecting 30-50% of treated patients. In addition to Cyclophosphamide-- doxorubicin, taxanes, and vincristine also generally cause the most profound alopecia.
Toxicity associated with anticancer drugs often forces discontinuation of treatment which may negatively impact the prognosis of a patient's condition and clinical outcome and result in compromising the quality of life. These toxicities are clinically managed by drug dose reduction or delay of treatment. Previous attempts to reduce chemotherapy associated alopecia, such as by using cold caps, have met with limited success. Thus, it is critical to develop new approaches that can selectively prevent the toxic side effects of these drugs with potential increase for therapeutic efficacy.
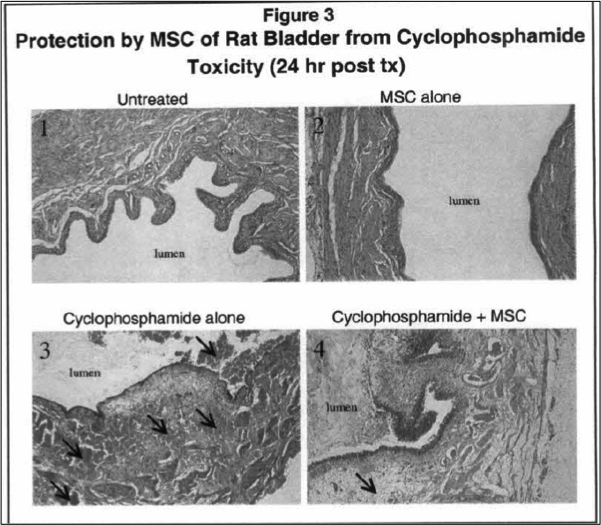
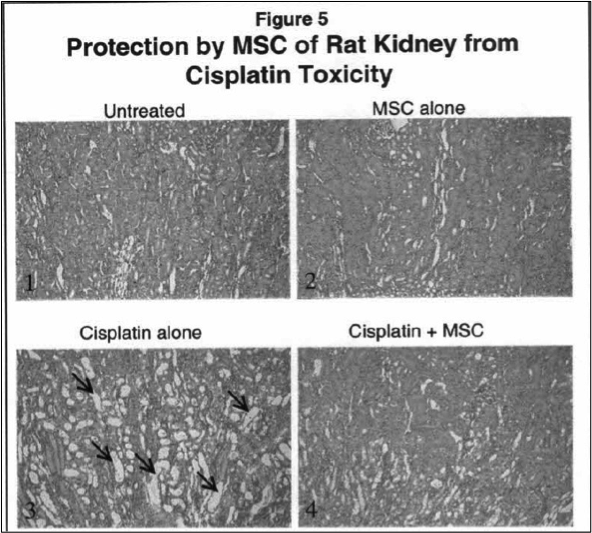
Researchers at Roswell Park Comprehensive Cancer Center have demonstrated that selenium containing compounds are highly effective in preventing alopecia and severe bladder toxicity associated with cyclophosphamide as well as in preventing kidney toxicity associated with cisplatin. In addition, the significant increase in creatinine and blood urea nitrogen (BUN) following treatment with cisplatin were restored to normal values in animals that were treated.
The present invention discloses a method for reducing toxicity of cyclophosphamide including alopecia and bladder toxicity. The method comprises administering to an individual, in need of treatment, cyclophosphamide and a selenium compound. The selenium compounds may be administered before, during or after administration of the anti- cancer agent. In one embodiment, the selenium compound is administered prior to chemotherapy and may be continued during and after the chemotherapy. By combining chemotherapy with the administration of selenium compounds, some of the side effects of cyclophosphamide such as weight loss, alopecia and bladder toxicity can be decreased.