Chronic myeloid leukemia (CML) accounts for approximately 20% of leukemia diagnosed in adults. The hallmark of CML is bcr-abl fusion gene, which arises from the reciprocal chromosomal translocation t(9:22)(q34;q11) resulting in the Philadelphia chromosome. The Bcr-Abl fusion protein exhibits deregulated tyrosine kinase activity that is necessary for the induction of CML.
Current targeted therapies all fundamentally function as signal transduction inhibitors of specific signaling pathways that are involved in the pro-survival/anti-differentiation aspect of cancer cells. The prototypic example of this is the fusion oncogene Bcr-Abl, which is expressed in both CML and acute lymphocytic leukemia (ALL). Bcr-Abl has unregulated Abl kinase activity, which is essential for CML blasts (although not ALL blasts) to survive and proliferate. The kinase inhibitor imatinib (Gleevec) is an Abl-specific inhibitor that was first-in-class as a tyrosine kinase “targeted” inhibitor, has great efficacy in CML, and has fundamentally changed how we treat disease.
However, CML appears to be the exception and not the rule, as many other cancers (including Bcr-Abl + ALL) have abnormal oncogenic signaling, yet inhibiting these pathways does not affect the cancer cell – primarily because the cancer does not actually need the signaling pathway for its survival. Thus, there is an ongoing unmet need for therapeutic cancer interventions that can exploit endogenous cancer cell specific genes without reliance on inhibiting the function of such genes.
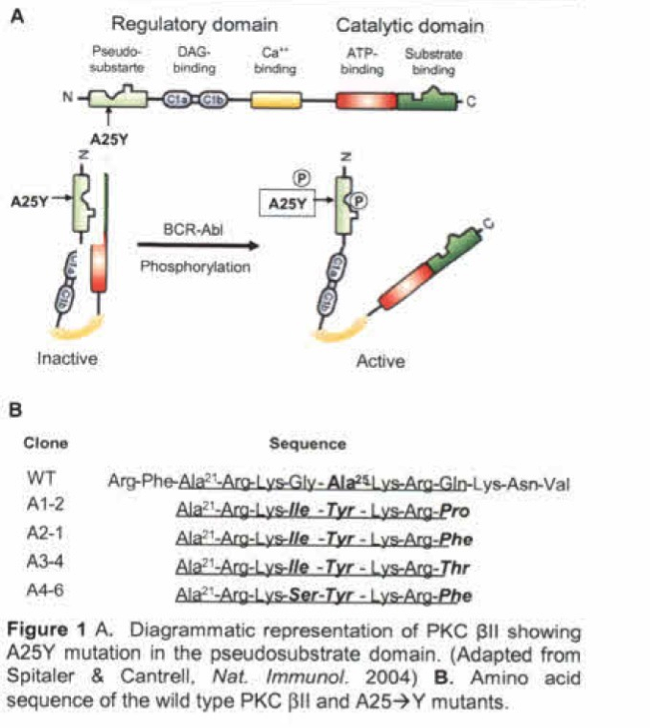
The proposed method comprises administering to an individual in need of treatment a “prodrug”(e.g., Protein Kinase C [PKC]), wherein the prodrug is a substrate for an enzyme, where the enzyme is endogenous to the cancer cells, and where the enzyme is preferentially or exclusively expressed by the cancer cells relative to non-cancer cells. The enzyme that is preferentially or exclusively expressed by the cancer cells relative to non-cancer cells is accordingly considered to be a cancer cell specific enzyme. Conversion of the prodrug to the active drug by the cancer cell specific enzyme results in inhibition of the growth of cancer cells.
This strategy is not dependent on the cancer cell actually needing the pathway to survive (unlike inhibitor strategies), but simply that the pathway is on. The “prodrug” that is specifically activated by abnormal kinase signaling, and in cancer cells is actively converted into a “suicide agent” that then kills the cell.