Dysregulated hematopoiesis in triple negative breast cancer
In contrast to our studies in MDS that focus on decreased production of immune-activating cells, our research in breast cancer examines excessive production of immune-suppressive cells.
This work studies how dysregulated hematopoiesis leads to the development of an immunologic milieu that is unable to support successful immunotherapy.
We are particularly interested in helping patients with triple negative breast cancer (TNBC). TNBC is characterized by a lack of receptors for estrogen, progesterone and epidermal growth factor and so drugs that target these receptors, which are used in other types of breast cancer, will not work.
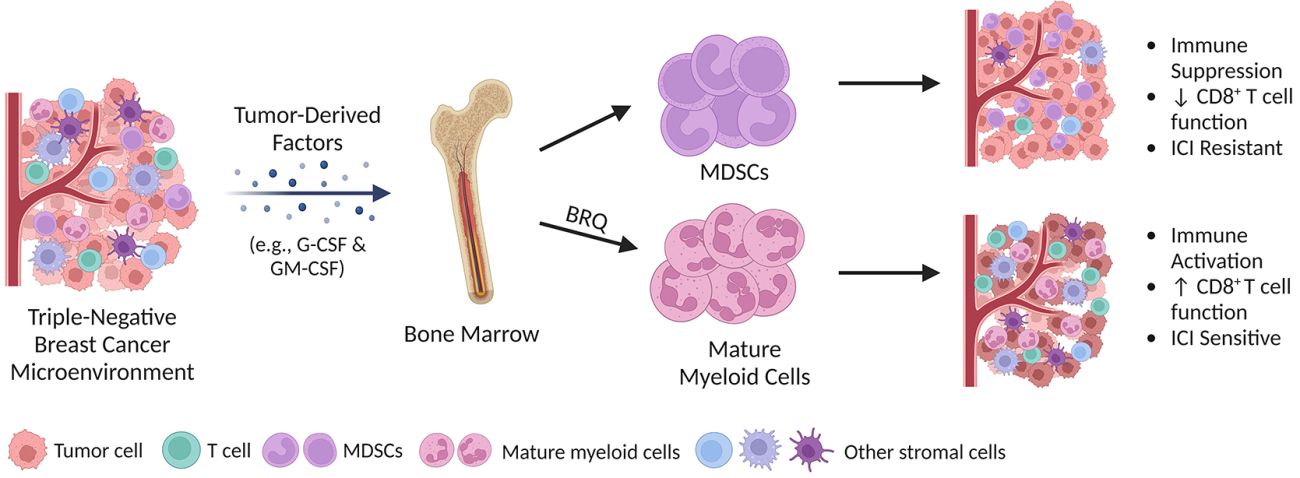
While new drugs that harness the patient’s own immune system, such as immune checkpoint inhibitors, have considerable promise for the treatment of TNBC, a barrier to the success of immune therapy in TNBC is that the tumor promotes the production of immune suppressive cells called myeloid-derived suppressor cells (MDSCs).
In TNBC, and other types of cancer, MDSCs expand their numbers and are persistent, resulting in the suppression of anti-tumor immune responses. Thus, any attempt to use the immune system to kill cancer cells would be enhanced by the simultaneous elimination of MDSCs.
We are currently testing strategies to eliminate MDSCs by targeting them at their source in the bone marrow. As an example, we have recently re-purposed a compound currently being tested in clinical trials for acute myeloid leukemia called Brequinar (BRQ) that when combined with immune checkpoint inhibitors suppresses growth of TNBC tumors.
We believe that this research will result in novel combinations of immunotherapy for patients with TNBC. This work is being done in collaboration with Scott Abrams, PhD, in the Department of Immunology at Rowell Park Comprehensive Cancer Center.
Inhibiting the biogenesis of myeloid-derived suppressor cells enhances immunotherapy efficacy against mammary tumor progression
- Journal: Journal of Clinical Investigaition, December 2022
- Authors: Colligan SH, Amitrano AM, Zollo RA, Peresie J, Kramer ED, Morreale B, Barbi J, Singh PK, Yu H, Wang J, Opyrchal M, Sykes DB, Nemeth MJ, Abrams SI.
- Citation: J Clin Invest. 2022 Dec 1;132(23):e158661. doi: 10.1172/JCI158661.
Connect with the Nemeth Lab
Email: Michael.Nemeth@RoswellPark.org
Phone: 716-845-1775
Department of Immunology
Roswell Park Comprehensive Cancer Center
Elm and Carlton Streets
Buffalo, NY 14263