The prostate tumor microenvironment is immune therapy suppressive
Advanced and recurrent prostate cancer are typically treated by blocking androgen receptor (AR) signaling, but these therapies rarely cure prostate cancer, and resistant tumors are eventually lethal. Thus, there is a need for additional therapies for prostate cancers.
Prostate cancers are marked by three key characteristics of the immune-excluded phenotype that reduce response to CPI therapy:
- Immuno-suppressive cell populations
- “Exhausted” T cells
- Chemokine/cytokine profiles that promote immunosuppression and impede CD8+ T cell infiltration
However, a study by our collaborators of over 200 mostly high-risk human resection specimens reported that prostate tumors do indeed contain tumor infiltrating cytolytic T-lymphocytes (TILs) and that the TIL density correlates with prognosis. These data suggest that human prostate cancer is marked by a variably immunosuppressive tumor immune microenvironment (TiME).
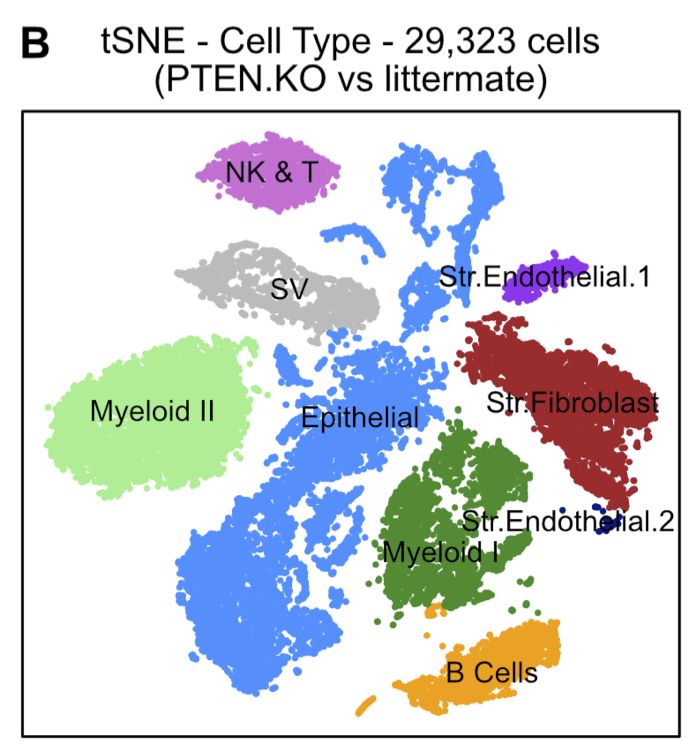
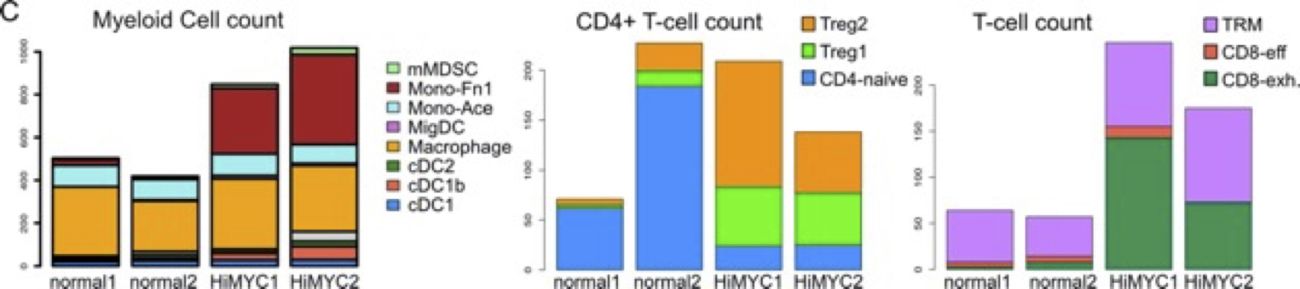
Moreover, using single-cell RNAseq to analyze two genetically engineered mouse models of prostate cancer, we found the specimens had been infiltrated with immunosuppressive myeloid populations and regulatory T cells (Tregs), accompanied by a 3X-10X increase in exhausted T cells.
One potential therapeutic pathway to reduce immune suppression is to interfere with immuno-suppressive cell infiltration into tumor, thereby enabling effector T cells to attack tumor.
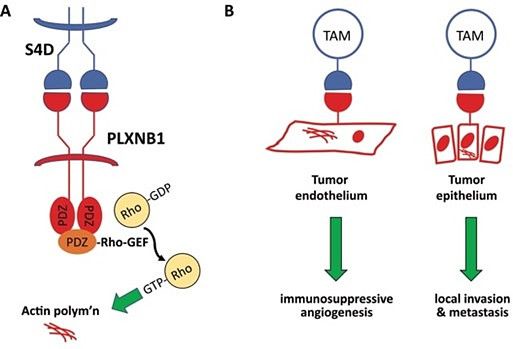
In both mice and humans, anti-SEMA4D has been reported to both reduce suppressive myeloid populations and Tregs, and to increase CD8+ T cells within the TiME, and in mouse models, to synergize with checkpoint inhibitor immune therapies to inhibit tumor growth.
We hypothesize that SEMA4D acts on the tumor microvasculature, disrupting the angiogenic-immunosuppressive axis that dictates immune cell trafficking into the tumor microenvironment.
We are therefore investigating the effects of SEMA4D signaling on the immunosuppressive-angiogenic axis, as well as measuring changes in tumor immunophenotype and transcriptomically defined TiME functional populations following anti-SEMA4D +/- immune therapy in our mouse models of prostate cancer.
We are also using functional imaging of prostate tumors in anti-SEMA4D treated mice to characterize changes in vascular permeability and vascular function.
Androgen deprivation exacerbates suppressive prostate cancer TiME
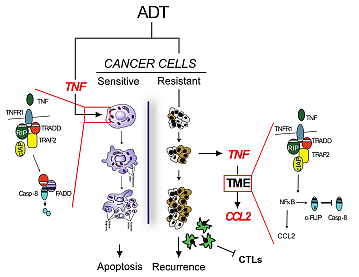
ADT (via castration) causes the prostate tumor volume to shrink in mouse prostate cancer models, but most tumors recur within 5-10 weeks. In earlier studies, we found TNF was induced by castration and was necessary for castration-induced tumor regression.
To investigate the mechanisms controlling the response to castration and recurrence, we blocked TNF signaling after castration-induced regression was underway and found a ‘phenotype switching.’ The tumors regress but recurrence is prevented, implying that the late wave of TNF secretion within the tumor drives recurrence.
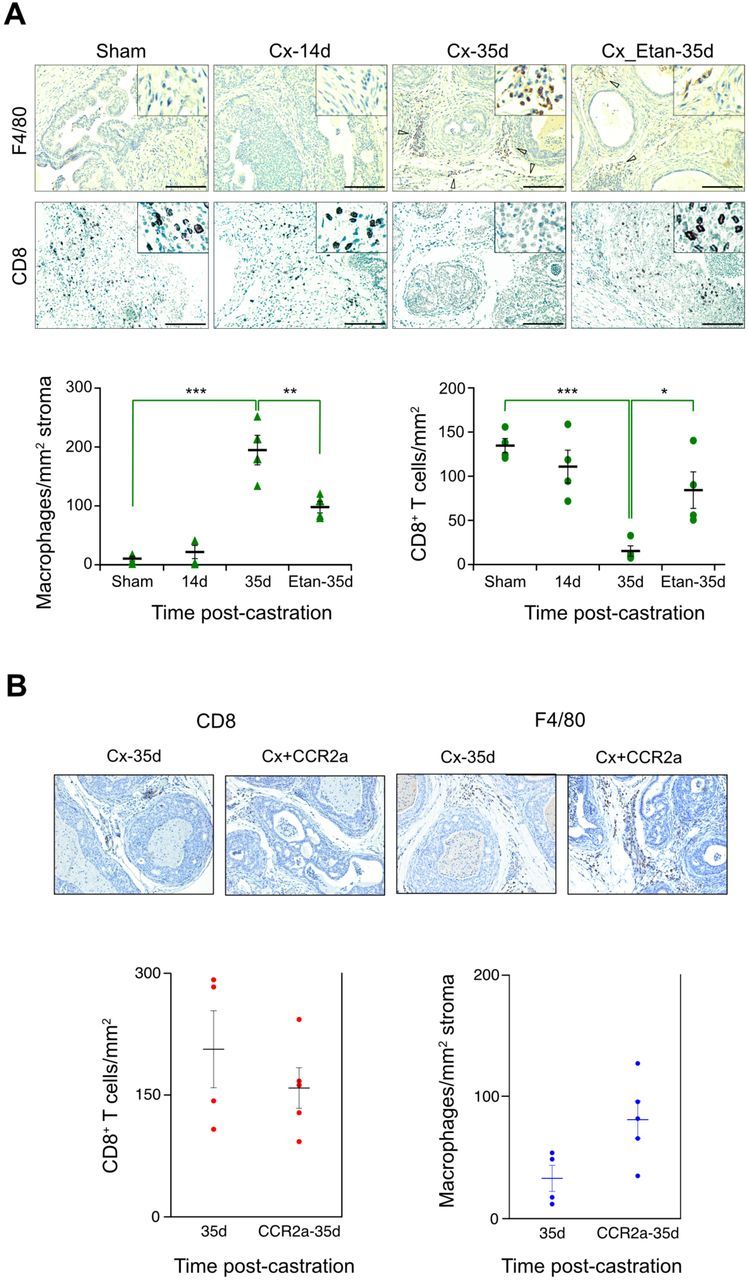
Late TNF secretion coincides with the expression of NFkB-regulated genes. The inhibition of signaling downstream of one NFkB-regulated protein – chemokine C-C motif ligand 2 (CCL2) – prevents post-castration tumor recurrence, effectively phenocopying post-castration TNF signaling blockade.
CCL2 was originally identified as a macrophage chemoattractant and indeed at late times after castration gene sets related to chemotaxis and migration are up-regulated. Importantly, enhanced CCL2 signaling during the tumor recurrence phase coincides with an increase in pro-tumorigenic macrophages and a decrease in CD8 T cells, suggesting that recurrence is driven at least in part by tumor immunosuppression.
Inflammation drives hyperplastic growth in BPH models
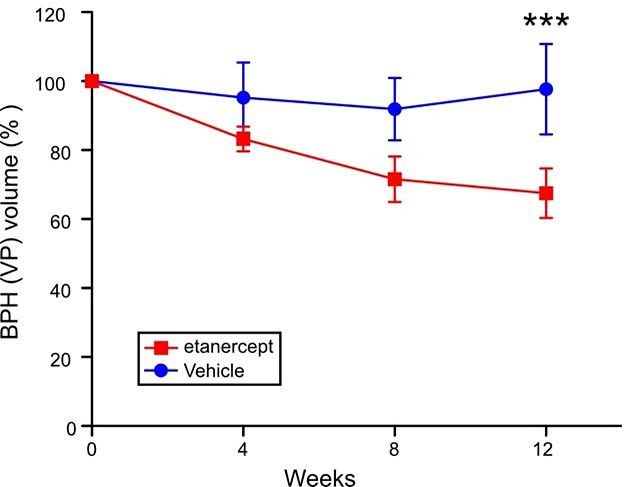
TNF is also a driver of inflammation in a related non-cancer prostate disease, benign prostatic hyperplasia (BPH). Using specific TNF blocking drugs, we showed TNF plays a causative role in the genesis and maintenance of BPH in mouse models.
This role of TNF was confirmed by dissecting anti-inflammatory drug usage in correlative studies of a large clinical cohort.
See the science
- Sha K, Zhang R, Maolake A, Singh S, Chatta G, Eng KH, Nastiuk KL, Krolewski JJ. Androgen deprivation triggers a cytokine signaling switch to induce immune suppression and prostate cancer recurrence. bioRxiv [Preprint]. 2024 Feb 18:2023.12.01.569685. doi: 10.1101/2023.12.01.569685.
- Zhang R, Singh S, Pan C, Xu B, Kindblom J, Eng KH, Krolewski JJ, Nastiuk KL. Rate of castration-induced prostate stroma regression is reduced in a mouse model of benign prostatic hyperplasia. Am J Clin Exp Urol. 2023 Feb 25;11(1):12-26. PMID: 36923722; PMCID: PMC10009314.
- Vickman RE, Aaron-Brooks L, Zhang R, Lanman NA, Lapin B, Gil V, Greenberg M, Sasaki T, Cresswell GM, Broman MM, Paez JS, Petkewicz J, Talaty P, Helfand BT, Glaser AP, Wang CH, Franco OE, Ratliff TL, Nastiuk KL, Crawford SE, Hayward SW. TNF is a potential therapeutic target to suppress prostatic inflammation and hyperplasia in autoimmune disease. Nat Commun. 2022 Apr 19;13(1):2133. doi: 10.1038/s41467-022-29719-1.
- Krolewski JJ, Singh S, Sha K, Jaiswal N, Turowski SG, Pan C, Rich LJ, Seshadri M, Nastiuk KL. TNF Signaling Is Required for Castration-Induced Vascular Damage Preceding Prostate Cancer Regression. Cancers (Basel). 2022 Dec 7;14(24):6020. doi: 10.3390/cancers14246020.
Connect with the Nastiuk Lab
Department of Cancer Genetics & Genomics
Roswell Park Comprehensive Cancer Center
Elm and Carlton Streets
Buffalo, NY 14263