G-protein Coupled Receptors as cancer drug targets
Composed of over 900 members in humans, G-protein Coupled Receptors (GPCRs) are seven-transmembrane proteins that regulate many physiological processes, including vision, olfaction, taste, behavior, and autonomic nervous system transmission.
GPCRs transduce extracellular signals from a variety of ligands through the plasma membrane, resulting in the modulation of intracellular signaling pathways. This is accomplished in large measure by the activation of heterotrimeric G-proteins and downstream second messengers.
The wide array of functions has resulted in the extensive utilization of GPCR targeted therapeutics, accounting for 30-50% of all currently used drugs.
Extensive use of GPCR drugs can also be attributed to GPCR localization on the cell surface, abrogating the requirement for a drug to be cell permeable, as well as the ability of GPCRs to bind a variety of ligands, including antibodies, peptides and small molecules.
In addition, GPCR signaling can be tightly regulated through the utilization of agonists, antagonists and inverse agonists.
Finding and evaluating high-priority GPCR targets
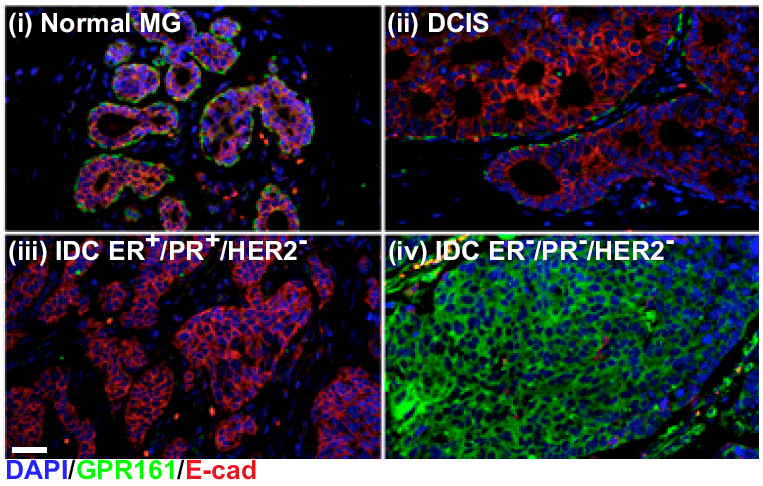
Drugs targeting GPCRs are rarely utilized in cancer treatment, despite evidence that GPCRs mediate many aspects of tumorigenesis, including cell proliferation, invasion, immune cell recruitment and secondary niche generation.
Genomic analyses have uncovered GPCR mutations, copy number alterations, and gene expression and methylation changes in a wide variety of cancers.
We hypothesize that determining the biological implication of these genomic alterations will allow utilization of GPCR targeted therapeutics in those patients with GPCR-driven tumors.
Our lab uses computational methods to select high priority GPCR targets, followed by experimental validation and therapeutic evaluation in three-dimensional cell culture and mouse models.
Recent publications
Cornwell AC and Feigin ME. Unintended effects of GPCR-targeted drugs on the cancer phenotype. Trends Pharmacol Sci. 2020 Dec;41(12):1006-1022. doi: 10.1016/j.tips.2020.10.001. Epub 2020 Oct 22. PMID: 33198923 PMCID: PMC7672258.
Insel PA, Sriram K, Wiley SZ, Wilderman A, Katakia T, McCann T, Yokouchi H, Zhang L, Corriden R, Liu D, Feigin ME, French RP, Lowy AM, Murray F. GPCRomics: GPCR expression in cancer cells and tumors identifies new, potential biomarkers and therapeutic targets. Front Pharmacol. 2018 May 22;9:431. doi: 10.3389/fphar.2018.00431. eCollection 2018. PMID: 29872392. PMCID: PMC5972277.
Feigin ME, Xue B, Hammell MC, Muthuswamy SK. G-protein-coupled receptor GPR161 is overexpressed in breast cancer and is a promoter of cell proliferation and invasion. Proc Natl Acad Sci USA. 2014 Mar 18;111(11):4191-6. doi: 10.1073/pnas.1320239111. Epub 2014 Mar 5. PMID: 24599592. PMCID: PMC3964064.
Feigin ME. Harnessing the genome for characterization of GPCRs in cancer pathogenesis. FEBS J. 2013 Oct;280(19):4729-38. doi: 10.1111/febs.12473. Epub 2013 Sep 2. PMID: 23927072. PMCID: PMC4283816.