Integrating immunotherapy and targeted therapy for ovarian and prostate cancer
My current research focuses on the genetic and epigenetic features of cancer cells, the interactions between cancer cells and immunosuppressive tumor microenvironment, and the development of mechanism-based innovative targeted therapies and immunotherapies to be translated into clinical practice to improve the outcomes for cancer patients.
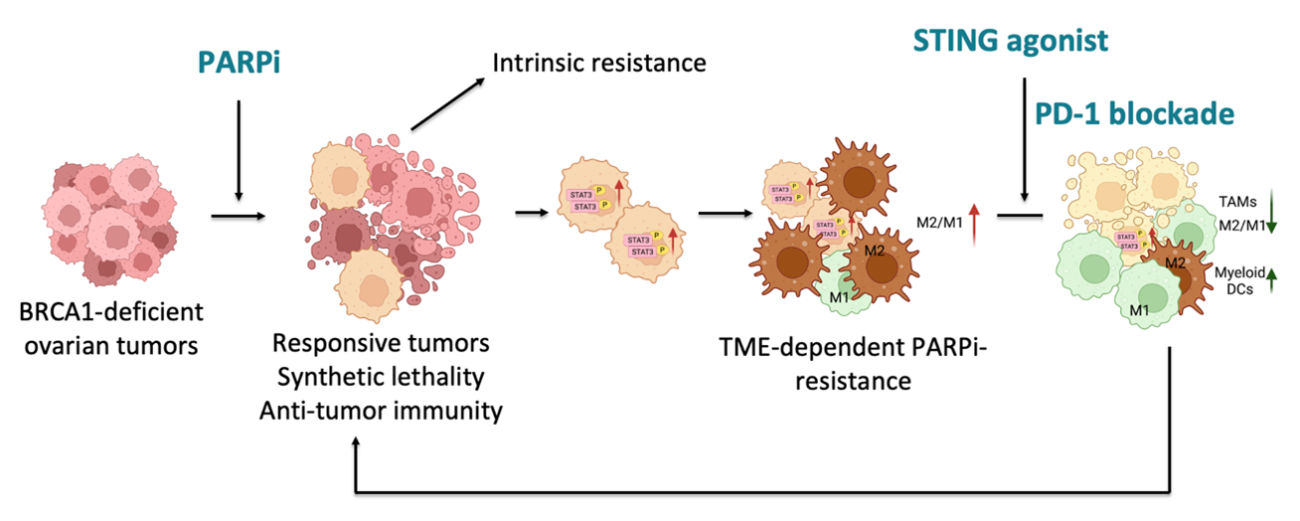
Targeting the therapeutic resistance of PARP inhibitors in BRCA-deficient ovarian cancer
High-grade serous ovarian cancer (HGSOC) accounts for over 70% of ovarian cancer deaths. My research on ovarian cancer has focused on the molecular mechanisms of Poly ADP ribose polymerase inhibitor (PARPi) therapy, both singly and in combination with immunotherapy.
PARPi have shown promising clinical activities for patients with BRCA mutations or other features of homologous recombination deficiency (HRD) and are changing the landscape of ovarian cancer treatment. However, the effects of PARP inhibition on the interaction of tumor cells with the tumor microenvironment and the host immune system remain unclear.
By using our in-house generated genetically engineered mouse models (GEMMs) of HGSOC, we found that, in addition to synthetic lethality, PARP inhibition also triggers robust local and systemic antitumor immunity involving both adaptive and innate immune responses in a STING-dependent manner.
Our recent work reveals an adaptive immunosuppression mechanism that confers resistance to PARPi in BRCA1-mutant ovarian tumors. This resistance is mediated by the enrichment of protumor polarization of tumor-associated macrophages (TAMs), driven by PARPi-induced activation of the STAT3 signaling pathway in tumor cells.
We further developed a strategy to overcome PARPi resistance by targeting both the tumor and the microenvironment in BRCA1-deficient ovarian cancer
Characterizing and targeting immunosuppression induced by tumor-intrinsic oncogenic signaling in HGSOC
Amplification and overexpression of CCNE1 (Cyclin E1) and PIK3CA (p110α) is frequently found in ovarian cancer without HRD and is associated with primary chemoresistance and poor clinical outcomes. These types of tumors have a low probability of responding to PARP inhibitors.
Our research employs syngenetic mouse models and humanized PDX models to investigate the role of Cyclin E1 and p110α in modulating the tumor immune microenvironment and therapeutic resistance. We aim to develop mechanism-based therapies for these types of ovarian cancer.
Novel combination therapies for PTEN-deficient CRPC
Prostate cancer is the most frequently diagnosed malignancy among males, with castration-resistant prostate cancer (CRPC) representing a particularly lethal manifestation. CRPC usually develops following androgen-deprivation therapy (ADT) for advanced metastatic disease. PTEN loss is commonly observed in aggressive stages of prostate cancer, including CRPC and metastatic cancer, affecting 40–60% of cases.
Our research explores the genetic and epigenetic characteristics of both the tumor and its immune microenvironment. We are developing innovative combination therapies for PTEN-deficient CRPC.
Identifying epigenetic regulators of suppressive myeloid cells for cancer treatment
The tissue microenvironment plays a crucial role in influencing various immunosuppression mechanisms that affect tumor growth. By employing syngeneic and humanized cancer models that mimic essential tumorigenic and immunogenic aspects, along with cutting-edge molecular and cellular technologies, our goal is to uncover the epigenetic regulators that are pivotal in myeloid cell differentiation and maturation during ovarian and prostate cancer development and progression.
This investigation aims to deepen our understanding of myeloid cell-mediated immunosuppression in cancers and potentially provide insights into novel immunotherapeutic strategies for cancer treatment.
Connect with the Ding Lab
Email: Liya.Ding@RoswellPark.org
Office location: Center for Genetics & Pharmacology (CGP) L5-310
Lab Location: Center for Genetics & Pharmacology (CGP) L5-111
Department of Immunology
Roswell Park Comprehensive Cancer Center
Elm and Carlton Streets
Buffalo, NY 14263