Landmark research: ERα-p53 Interaction in Cancer Cell Proliferation
Estrogen Receptor alpha (ERα/ESR1) and tumor suppressor protein p53 have diametrically opposite roles in regulating cellular proliferation. In normal physiology, homeostatic balance between these opposing cellular regulators ensures optimal functioning for the organism. Loss of such finite balance could lead to various diseases including cancer.
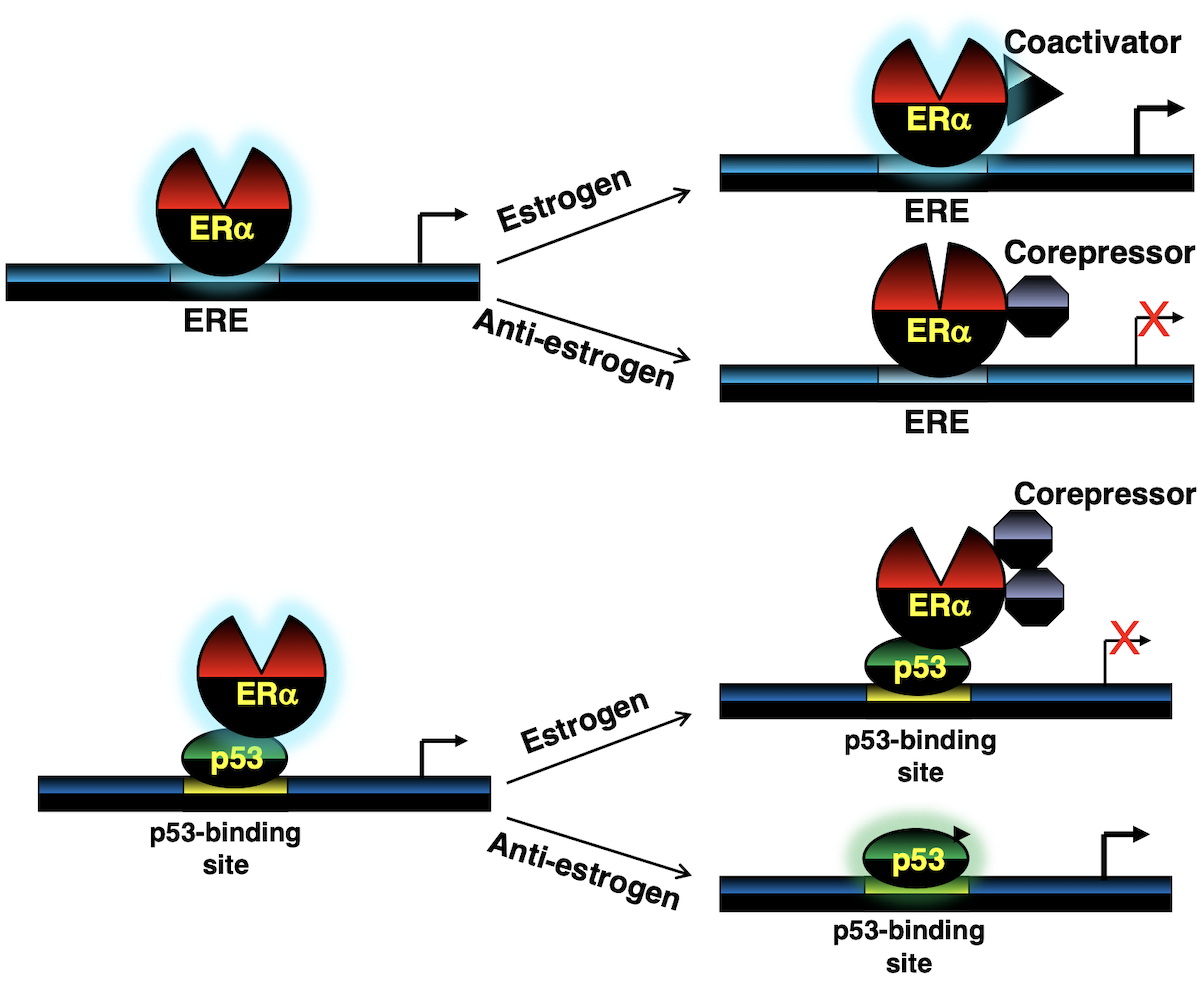
The canonical view is that estrogen binds to estrogen receptors and changes the conformation of the protein enabling it to bind estrogen response elements (EREs) on target gene promoters leading to activation of the receptor and ensuing expression of pro-proliferation genes.
Mechanisms of estrogen receptor antagonism toward p53 and its implications in breast cancer therapeutic response and stem cell regulation
- Journal: Proceedings of the National Academy of Sciences, June 2010
- Authors: Konduri SD, Medisetty R, Liu W, Kaipparettu BA, Srivastava P, Brauch H, Fritz P, Swetzig WM, Gardner AE, Khan SA, Das GM.
- Citation: Proc Natl Acad Sci U S A. 2010 Aug 24;107(34):15081-6. doi: 10.1073/pnas.1009575107.
Analyzing a new mechanism in response to tamoxifen therapy in breast cancer patients
NCT01027416We discovered an alternate mechanism underlying ERα function in luminal breast cancer cells wherein it directly binds wild-type p53 on p53-target gene promoters leading co-repressor recruitment and repression of p53-target genes involved in inhibiting cellular proliferation.
Thus, ERα drives abnormal proliferation of cancer cells via two mechanisms: (i) the canonical mechanism where estrogen-bound ERα activates pro-proliferation genes, and (ii) ERα binds and functionally suppresses p53 thereby downregulating transcription of p53-target genes that are responsible for blocking cell proliferation (PNAS, 2010).
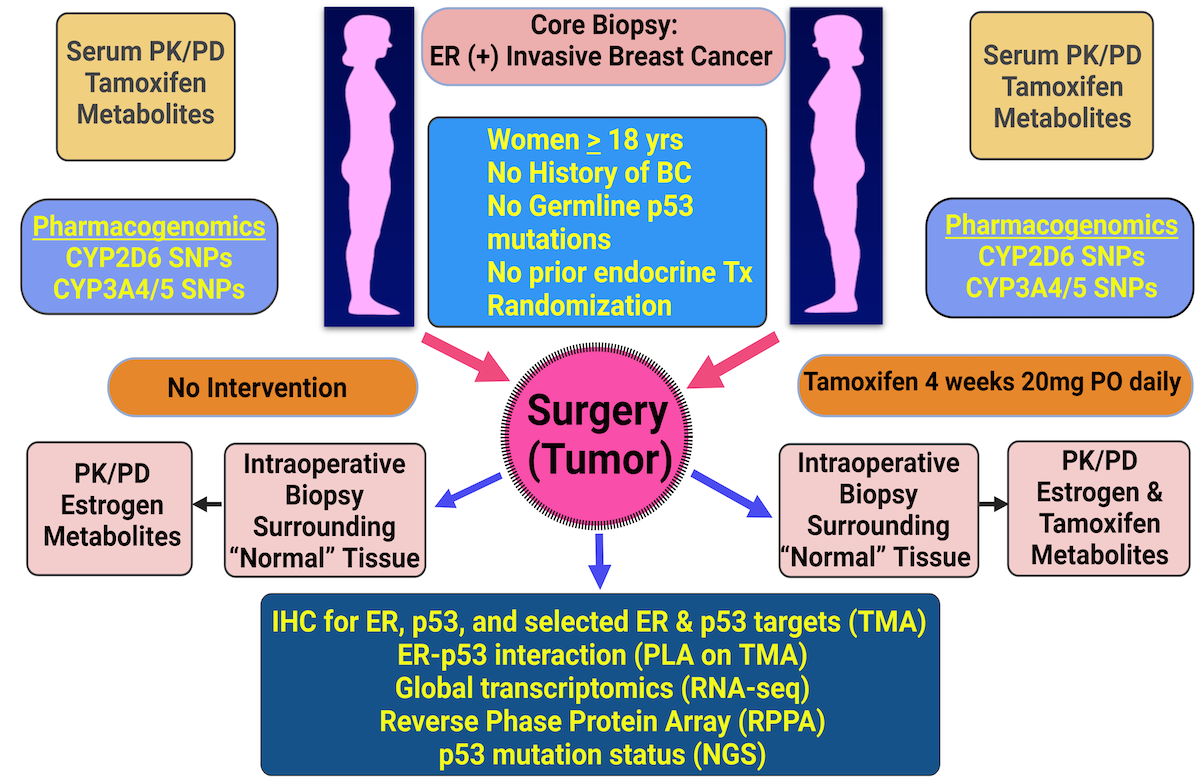
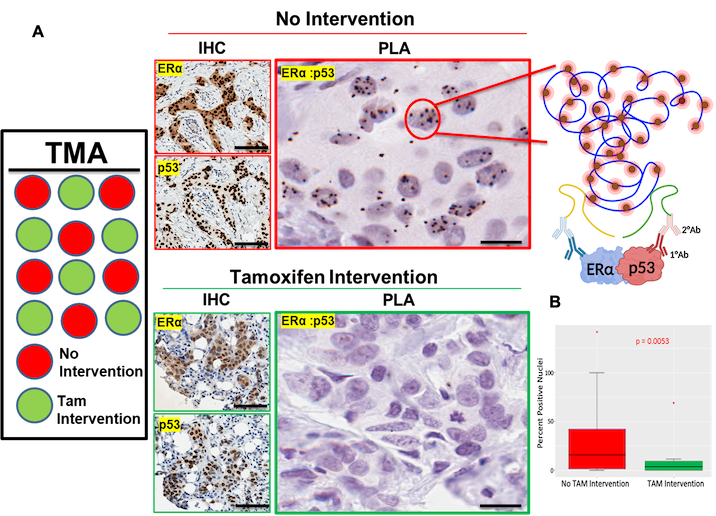
Consistent with our findings in breast cancer cell line models, in a randomized window-of-opportunity clinical trial in breast cancer patients with ERα-positive/wild-type p53 tumors, we observed that ERα-p53 interaction indeed occurs in vivo in the tumors and that interaction is disrupted in response to tamoxifen therapy leading to reactivation of p53 and reprogramming of gene expression (manuscript submitted).
These findings have important clinical implications in stratifying ERα-positive breast cancer patients to those who would and would not respond to tamoxifen therapy.
Contact the Das Lab
Email: Gokul.Das@RoswellPark.org
Phone: 716-845-8542
Department of Pharmacology and Therapeutics
Roswell Park Comprehensive Cancer Center
Elm and Carlton Streets
Buffalo, NY 14263