Novel therapeutics targeting p53 mutant cancers
Our research focuses on defining mechanisms of tumor progression and uncovering novel targets for treatment of metastatic tumors.
We take genetic and pharmacological approaches to decipher mechanisms mediating crosstalk in the tumor microenvironment, identify dysregulation of DNA repair mechanisms in cancer, and uncover novel targets that can be therapeutically targeted to improve patient survival.
We use state-of-the-art technologies such as genomics, CRISPR-mediated genetic perturbation, patient derived xenograft (PDX) models, and syngeneic tumor models, as well as molecular biology approaches.
Thymidine analog TAS102 plus PARP inhibitor inhibits growth of colon, pancreatic tumors
Working with co-author and Roswell Park colleague Christos Fountzilas, MD, FACP, the Bakin Lab investigated DNA repair upon challenge with thymidine analogs incorporated into genomic DNA during replication, hoping to better understand DNA differences in cancer and normal cells.
Roswell Park research funding
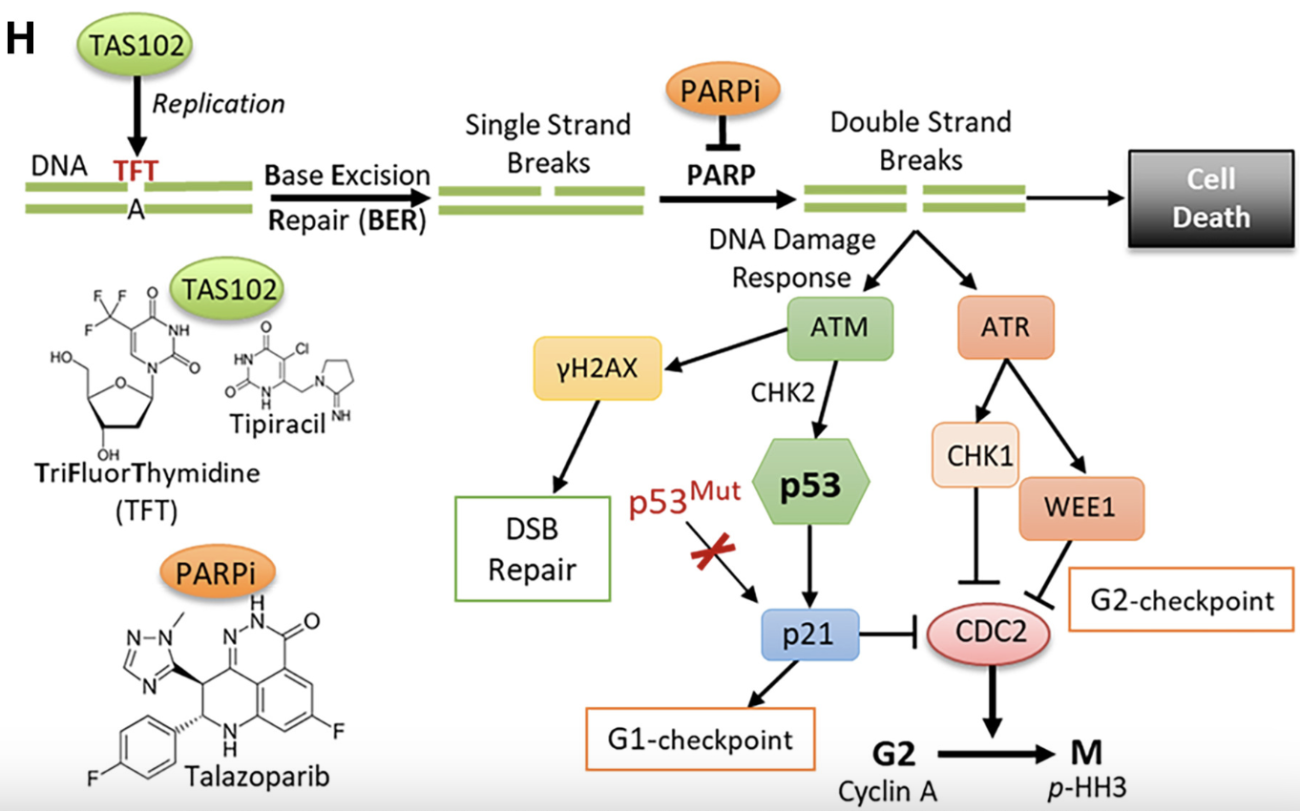
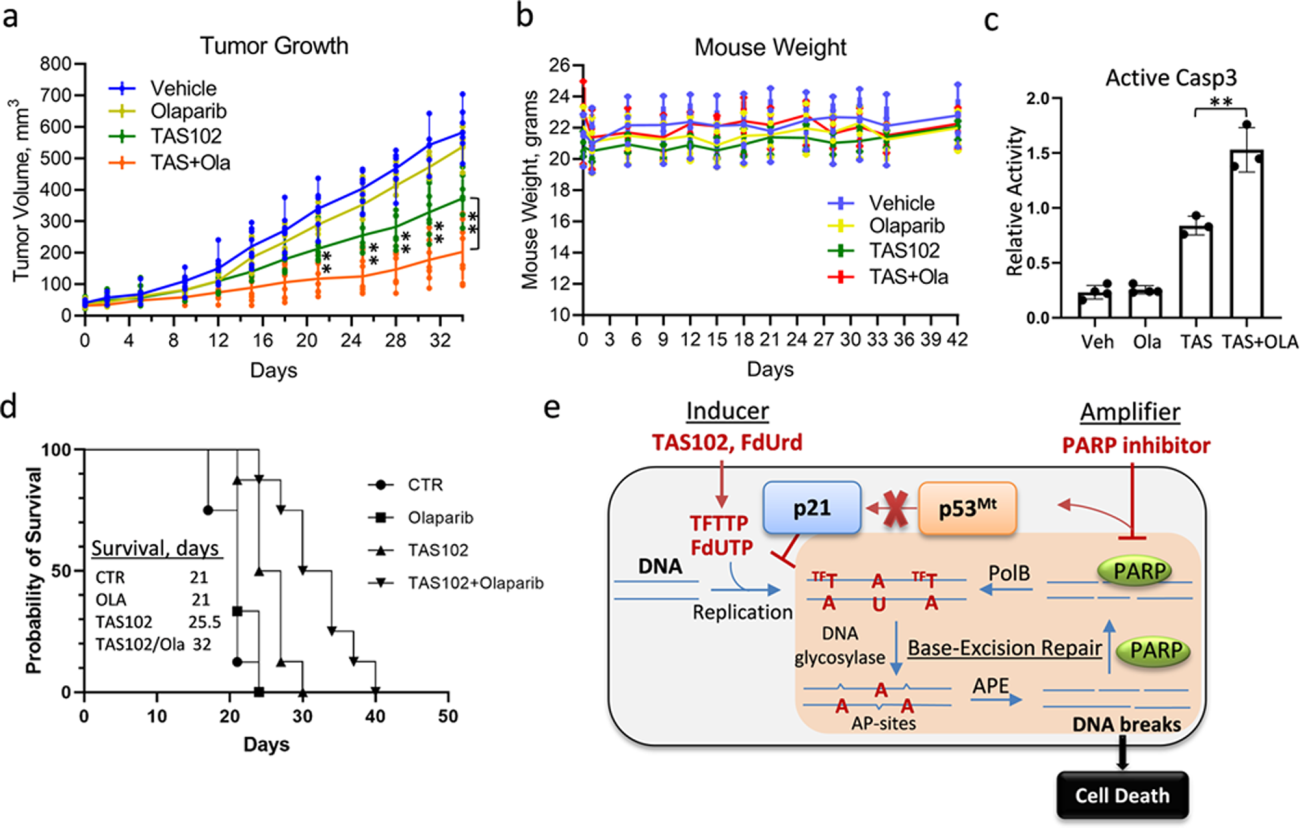
We discovered that p53-mutant cancer cells accumulate DNA breaks due to post-replicative removal of thymidine analogs. The data show that suppressing the PARP protein, which enables cancer cells to repair themselves, enhances DNA damage induced by thymidine analogs, leading to cancer cell death.
The two-drug combination — the thymidine analog trifluorothymidine TAS102 plus a PARP inhibitor — works together to inhibit the growth of colon and pancreatic cancers, including patient-derived tumor xenografts.
The two-drug combinatorial strategy is now being examined in the first-in-human phase 1 clinical study for refractory gastrointestinal cancers, and we are investigating the next series of drug combinations based on this initial success. Our findings could be extended to different cancer types beyond colorectal and pancreatic.
Dr. Bakin’s doctoral trainees Mohammed Alruwaili (first author) and Priyanka Rajan, along with former lab member Justin Zonneville, contributed to this groundbreaking study. Their work was featured in the NCI’s Cancer Currents blog.
PubMedFull text articlePress releaseNCI blog post
Phase I clinical trial
Trifluridine/ Tipiracil and Talazoparib for the Treatment of Patients with Locally Advanced or Metastatic Colorectal or Gastroesophageal Cancer
This phase I trial investigates the side effects and best dose of talazoparib when given together with trifluridine/tipiracil for the treatment of patients with colorectal or gastroesophageal cancer that has spread to nearby tissue or lymph nodes (locally advanced) or other places in the body (metastatic).
Targeted therapies for inducing DNA damage in p53-mutant triple negative breast cancer (TNBC) cells
p53 mutant cancers showed substantial dysregulation in base excision repair (BER). This defect rendered accumulation of DNA damage in p53 mutant cells upon treatment with deoxyuridine analogues.
Bakin Lab research funding
NCI R21 Exploratory/Developmental Grant
METAvivor Translational Research Award
Notably, inhibition of poly (ADP-ribose) polymerase (PARP) greatly enhanced this response, whereas normal cells responded with activation of the p53-p21 axis and cell cycle arrest. Inactivation of either p53 or p21/CDKN1A conferred the p53 mutant phenotype.
Preclinical animal studies demonstrated a greater anti-neoplastic efficacy of the drug combination (deoxyuridine analogue and PARP inhibitor) than either drug alone. This work illustrates a selective combination therapy strategy for p53 mutant cancers that will improve survival rates and treatment outcomes.
Current doctoral student Mohammed Alruwaili participated in this study, and former Bakin Lab master’s trainee Justin Zonneville contributed as first author. Their work was highlighted in the Springer Nature Research Communities Behind the Paper blog channel.
PubMedFull text articleBehind the Paper
Connect with the Bakin Lab
Department of Cancer Genetics & Genomics
Roswell Park Comprehensive Cancer Center
Elm and Carlton Streets
Buffalo, NY 14263