Identifying the novel drivers of PDAC malignancy
Pancreatic ductal adenocarcinoma (PDAC) is the most common form of pancreatic cancer and the deadliest major cancer with only a 12% 5-year survival rate. Many factors contributing to this lethality are aggressive, early metastatic spread coupled with late detection, poor response to conventional and targeted treatment options, and a limited number of known “drivers” that can be targeted to increase therapeutic efficacy.
Additionally, a subpopulation of cancer cells, known as cancer stem cells, are well known to promote therapeutic resistance and metastasis, but the drivers of cancer stem cells remain elusive as well. The Abel Lab is focused on identifying novel drivers of the malignancy programs of PDAC, including those that control pancreatic cancer stem cells.
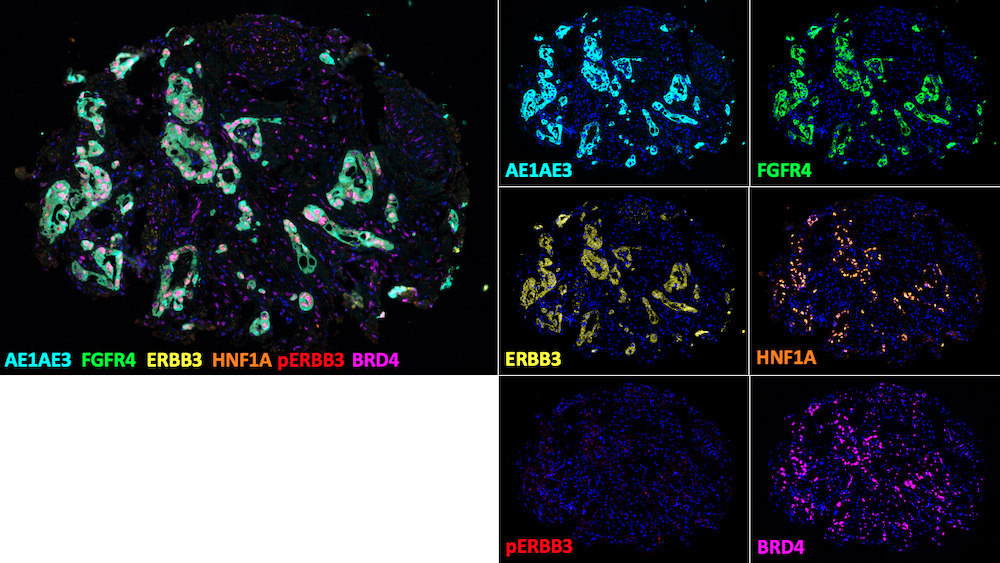
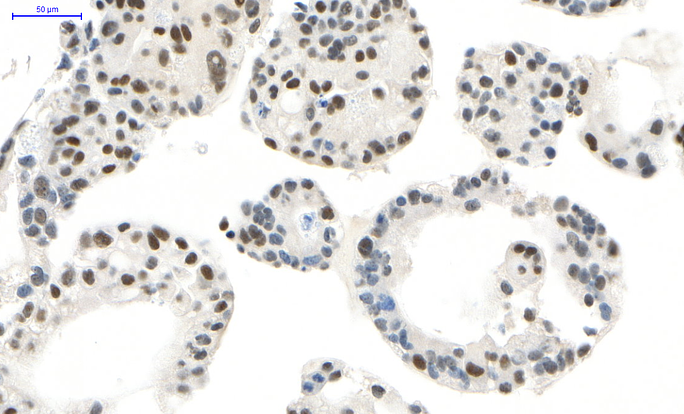
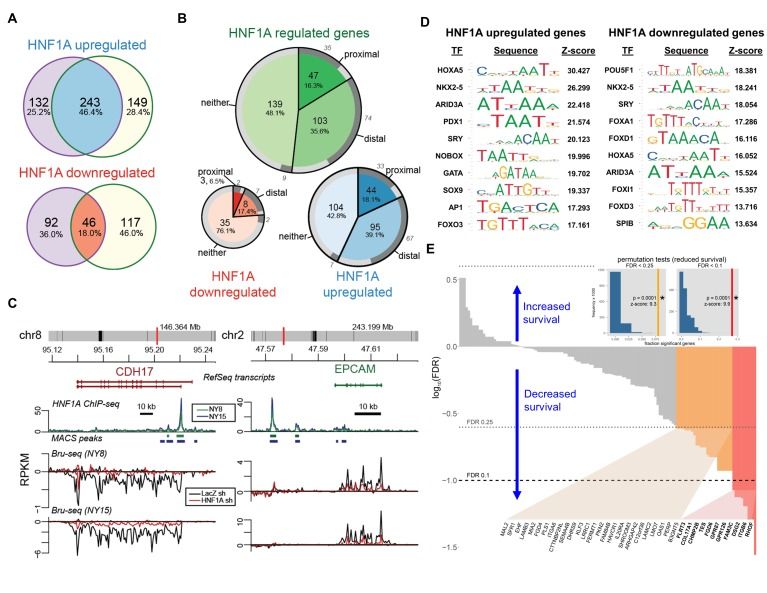
Much of our research is centered around characterizing the roles for the gastrointestinal-lineage transcription factor, HNF1A (hepatocyte nuclear factor 1-alpha) in PDAC. We previously identified HNF1A as a novel driver of pancreatic cancer stem cells and found that genetic targeting of HNF1A is capable of stripping these cells of their stemness, resulting in a potent reduction in tumor growth.
Ongoing studies in our lab have found that HNF1A responds dynamically to targeting the oncogene KRAS, which is mutated in >90% of PDAC and drives the disease. This response to KRAS-inhibition appears to be protective and allows PDAC cells to survive oncogene-ablation, and co-targeting these proteins appears synergistic.
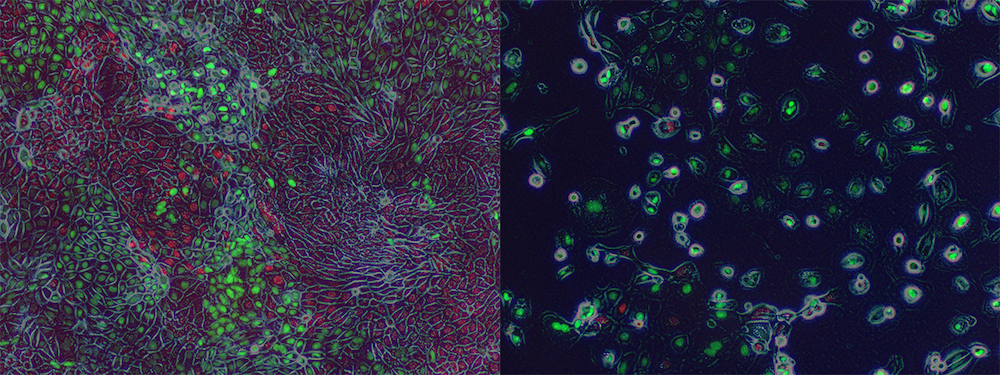
Additionally, we have found that HNF1A promotes a migratory and invasive phenotype in vitro and metastasis in vivo. Ongoing research in the lab is focused on understanding how HNF1A promotes this aggressive phenotype, with special attention paid to HNF1A-target genes FGFR4, Syndecan-1, and ERBB3.
As multiple facets of PDAC are regulated by HNF1A, we are also examining ways to block HNF1A’s expression and/or activity pharmacologically. We have found that HNF1A expression is directly regulated by the bromodomain and extraterminal (BET) protein BRD4 and that multiple BET-inhibitors potently ablate HNF1A expression.
Interestingly, the biological consequences of BET-inhibitor treatment (including growth arrest and lost stemness) are dependent upon the inhibition of HNF1A, and re-expression of HNF1A is able to reverse the effects of BET-inhibitors on PDAC cells. We have also developed genetic reporters for HNF1A’s transcriptional activity and are using these reporters to screen and identify novel inhibitors of HNF1A.
Our long-term goal is to identify, characterize, and target novel drivers of PDAC, much like HNF1A, which we believe will expand the efficacious treatment options for patients with PDAC and ultimately cure the disease.
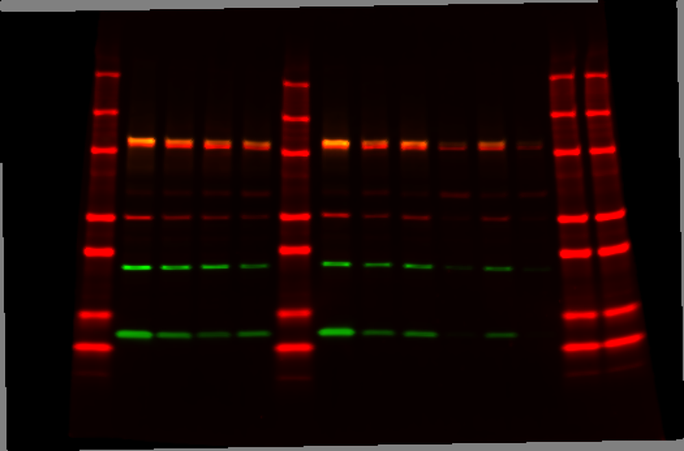
HNF1A is a novel oncogene that regulates human pancreatic cancer stem cell properties
- Publication: eLife, August 2018
- Authors: Abel EV, Goto M, Magnuson B, Abraham S, Ramanathan N, Hotaling E, Alaniz AA, Kumar-Sinha C, Dziubinski ML, Urs S, Wang L, Shi J, Waghray M, Ljungman M, Crawford HC, Simeone DM.
- Citation: Elife. 2018 Aug 3;7:e33947. doi: 10.7554/eLife.33947.
Contact us
Department of Molecular & Cellular Biology
Roswell Park Comprehensive Cancer Center
Elm and Carlton Streets
Buffalo, NY 14263