New Developments in Treatment
Brain tumors are relatively uncommon, affecting 200,000 Americans each year, and range from benign (not cancerous) to malignant (cancerous). Most brain tumors are secondary, which means the cancer spread to the brain from another area of the body, but a smaller number of cases involve primary tumors, which start in the brain tissue.
The most common and well-known type of primary brain cancer in adults is glioblastoma, which is a highly aggressive and difficult-to-treat disease. Much less is known about low-grade gliomas, a group of primary brain cancers that most often develop in younger people during the prime of life, with an average age at diagnosis of only 37 years.
What Is Low-Grade Glioma?
Low-grade gliomas start in glial cells, which are specialized “gluey” brain cells that support and protect the nervous system. Low-grade glioma tumors develop and progress slowly, but even a small, slow-growing tumor can cause big problems, especially if it’s located in a critical area of the brain.
Because these tumors grow slowly, the onset of symptoms is subtle and gradual, making the condition harder to recognize. Once tumors reach a certain size, symptoms such as headaches, nausea, loss of balance, weakness (particularly on one side of the body), neurologic deficits (such as difficulty concentrating or speaking), or seizures emerge.
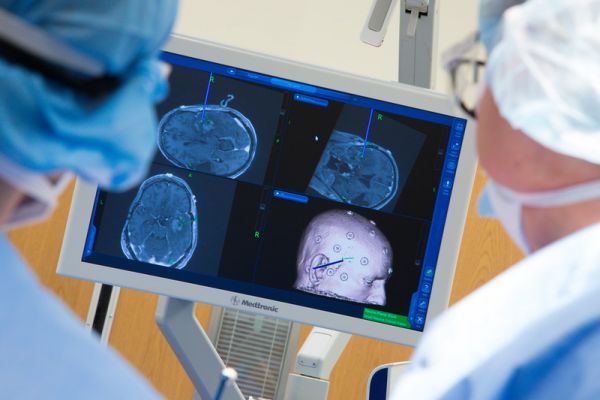
Unfortunately, low-grade gliomas tend to invade areas of the brain that are essential for carrying out important functions such as speech and movement. Before developing a treatment plan, it is often necessary to create a “map” of the critical language and motor regions of the brain that may be near or adjacent to the tumor.
What Is Brain Mapping?
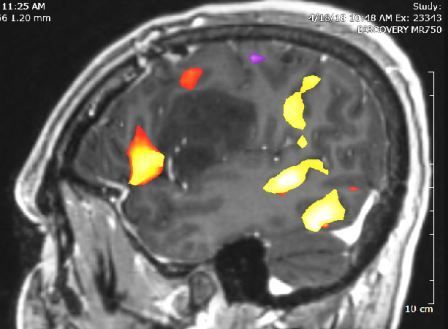
One safe and noninvasive brain-mapping technique used here at Roswell Park is called functional magnetic resonance imaging (fMRI), which detects changes in oxygen levels in the brain associated with blood flow.
When you’re using a part of your brain, the neurons in that area are firing and consuming energy. Blood flows to these firing neurons to feed them the oxygen they need to function, and fMRI measures the changes in oxygen and blood flow that occur with different kinds of brain activity and creates a detailed picture.
fMRI can show the various areas of the brain activated during speech or motor functions, but another brain-mapping technique that we use takes place while the patient is awake and speaking while the brain is exposed. This procedure is called an awake craniotomy.
In an awake craniotomy, the patient is initially fully sedated while the scalp is numbed with long-acting local anesthetic. After the brain is exposed, sedation is discontinued and the patient wakes up, though they will feel no pain (because the brain has no pain receptors). The “awake” portion of this procedure is necessary so that the brain can be mapped while a patient is speaking or performing a motor task, thereby minimizing the risk of affecting critical brain areas while the tumor is removed.
At Roswell Park, we’ve performed this technique for many years, although we do fewer awake craniotomies each year as fMRI technology advances.
Never miss another Cancer Talk blog!
Sign up to receive our monthly Cancer Talk e-newsletter.
Sign up!Advances in Glioma Treatment: Personalized Medicine and Immunotherapy
We used to diagnose and treat low-grade gliomas based on how these tumors looked under a microscope, but we’re now finding that microscopic appearance is far less important than genetic changes that have occurred in the tumor. If we can identify these changes, then we can predict how the tumor is going to behave and how aggressively we need to treat it. The most important genetic change that we look for in low-grade glioma is a mutation in the IDH (isocitrate dehydrogenase) gene. Patients with this mutation actually have a much better prognosis than those without it. What is particularly interesting about this “good” mutation is that it is present in every single cell in the tumor, which means that the mutation occurred very early in the course of the tumor’s development.
Another important mutation that we look for in low-grade glioma patients is codeletion, which occurs when two chromosomes (1 and 19) have been lost from tumor cells. Patients with codeletion respond significantly better to chemotherapy than those without this mutation, so we use this type of genetic information to guide and personalize treatment decisions every day.
The vast majority of patients with low-grade gliomas respond well to treatment. Surgery alone might be curative in some cases, eliminating both the tumor and any associated symptoms such as seizures. Radiation therapy and chemotherapy are also used for low-grade gliomas and they can be effective in many patients. Immunotherapy may be a treatment option for low-grade glioma patients in the future. Research conducted here at Roswell Park has led to the creation of an immunotherapy vaccine, SurVaxM, that targets a cancer-fueling protein called survivin. SurVaxM has shown promise in treating glioblastoma, the more common and aggressive form of primary brain cancer.