Specializing In:
- Hematopoiesis
- Leukemia
Positions
Roswell Park Comprehensive Cancer Center
- Assistant Professor of Oncology
- Co-Chair, Leukemia Translational Research Group
- Department of Immunology
Background
Education and Training
- 1996-2001 - PhD - Pharmacology and Toxicology - Dartmouth Medical School, Hanover, NH
Fellowship
- 2001-2008 - Post-Doctoral Fellow, Hematology, National Human Genome Research Institute, Bethesda, MD
Professional Memberships
- American Society of Hematology
Professional Experience
- 2008-Present - Departments of Medicine and Immunology, Roswell Park Comprehensive Cancer Center, Buffalo, NY
Research Overview
Myelodysplastic syndromes (MDS) are diseases of old stem cells characterized by failure of normal blood cell production. This leads to life-threatening symptoms such as anemia (low red blood cells), higher risk of infections (low white blood cells) and higher risk of bleeding (low platelets). For some patients, their disease will progress to the deadly disease Acute Myeloid Leukemia (AML) Current chemotherapy for these patients can improve their blood cell production, quality of life, and decrease the progression to AML. However, these effects are seen in only 50% of patients and when they occur, these effects are transient. We need new strategies to improve outcomes and quality of life for patients with MDS.
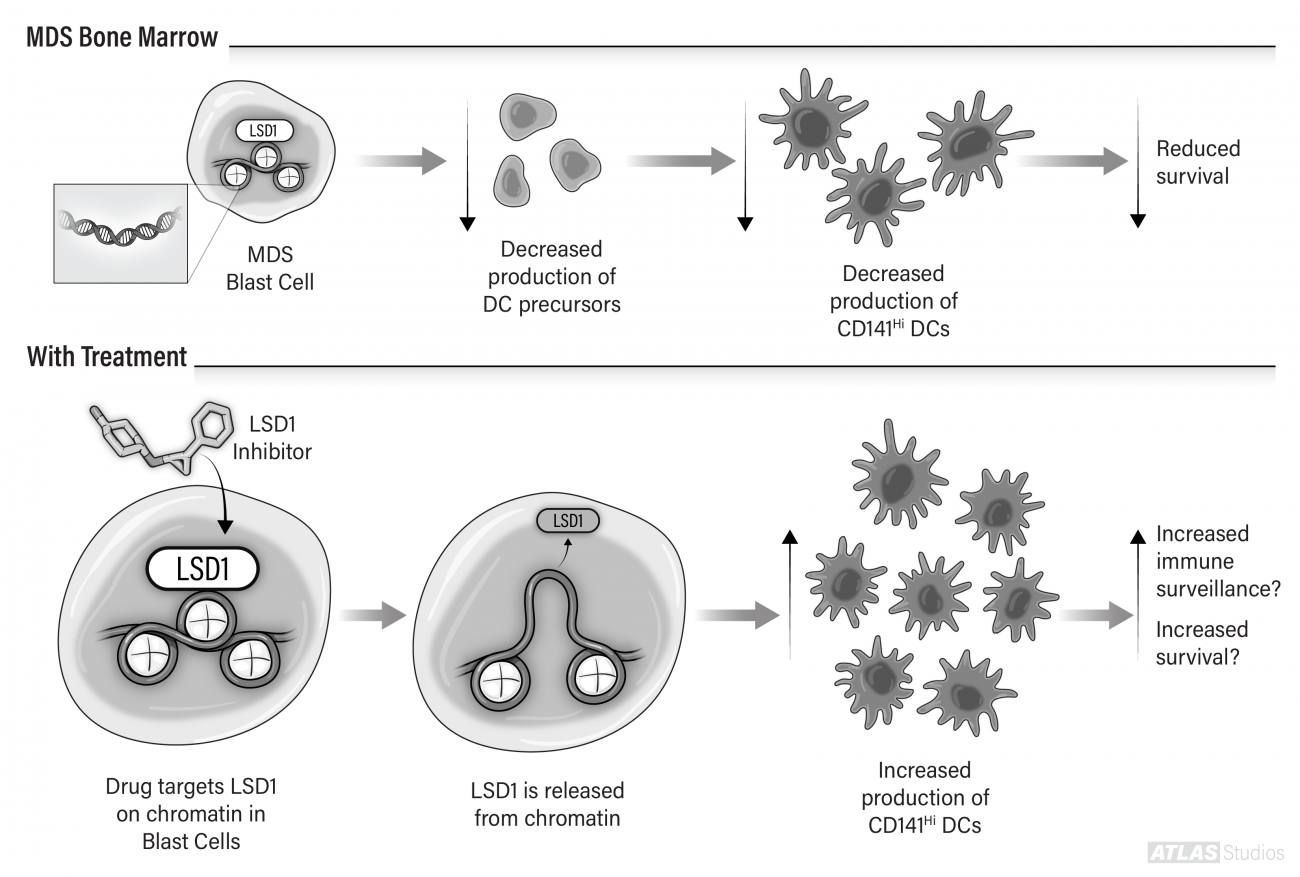
There is tremendous excitement about therapies that use the patient’s immune system to recognize and kill cancer cells – “immunotherapy”. Unfortunately, MDS patients make fewer mature immune cells and this is particularly true for high-risk patients. We have found that one type of immune cell that is defectively produced in MDS patients is called a dendritic cell (DC). DCs eat up dead and dying cells and use this material to educate the immune system to target and kill the cause of damage. We hypothesize that DCs are important immune cells for MDS patients and we have found that MDS patients with the fewest number of a particular type of DC (termed a CD141Hi DC) have the shortest survival (see figure).
In MDS, decreased production of DC precursors leads to decreased numbers of CD141Hi DCs, which is associated with reduced survival. LSD1 is a protein that binds to DNA and turns off genes. We have found that treating patients’ blast cells with a drug that inhibits LSD1 increases production of CD141Hi DCs. Future work will be done to determine if these cells can enhance the immune system to target MDS blasts (“immune surveillance”) and increase survival.
Our research projects are centered around answering the question - can we can use DCs to activate the patient’s immune system to kill their cancer cells? In one project, depicted in the Figure, we are testing drugs that cause the MDS blasts to turn into DCs in order to enhance the ability of the patient’s immune system to target MDS blasts. In another project, we are performing clinical trials in which we take advantage of the DC’s ability to educate the immune system and give patients a vaccine against proteins expressed by their MDS blast cells.
We are testing these concepts with our long-time collaborator Dr. Elizabeth Griffiths, a physician-scientist who directs the MDS Program at Roswell Park. The long-term goal of our research is to develop new ideas and strategies for using immunotherapy to treat patients with MDS.
If you are interested in learning more about our research, we would be happy to answer your questions.
Contact Information:
Dr. Michael J. Nemeth, PhD
Michael.Nemeth@Roswellpark.org
Office: 716-845-1775
If you would like to make a donation to support MDS research at Roswell Park, please contact Jenine Trzewieczynski at the Roswell Park Alliance Foundation.
Jenine M. Trzewieczynski
Major Gifts Officer
Roswell Park Alliance Foundation
Jenine.Trzewieczynski@roswellpark.org
Office:716-845-3872
View the Nemeth Lab
Publications
- Srivastava P, Tzetzo SL, Gomez EC, Eng KH, Jani Sait SN, Kuechle JB, Singh PK, De Jong K, Wiatrowski KR, Peresie J, Dimitroff A, Lynch ML, Wang J, Abrams SI, Griffiths EA, Nemeth MJ. Inhibition of LSD1 in MDS progenitors restores differentiation of CD141Hi conventional dendritic cells. Leukemia. 2020 Feb 25:10.1038/s41375-020-0765-5. Epub ahead of print. PMID: 32099035.
- Griffiths EA, Srivastava P, Matsuzaki J, Brumberger Z, Wang ES, Kocent J, Miller A, Roloff GW, Wong HY, Paluch BE, Lutgen-Dunckley LG, Martens BL, Odunsi K, Karpf AR, Hourigan CS, Nemeth MJ. NY- ESO-1 Vaccination in Combination with Decitabine Induces Antigen-Specific T-Lymphocyte Responses in Patients with Myelodysplastic Syndrome. Clin Cancer Res. 2018 Mar 1;24(5):1019-1029. doi: 10.1158/1078-0432.CCR-17-1792. Epub 2017 Sep 25. PMID: 28947565. PMCID: PMC5844797
- Srivastava P, Paluch BE, Matsuzaki J, James SR, Collamat-Lai G, Blagitko-Dorfs N, Ford LA, Naqash R, Lübbert M, Karpf AR, Nemeth MJ (Co-Senior Author), Griffiths EA. Induction of cancer testis antigen expression in circulating acute myeloid leukemia blasts following hypomethylating agent monotherapy. Oncotarget. 2016 Mar 15;7(11):12840-56. doi: 10.18632/oncotarget.7326 Epub 2016 Feb 11. PMID: 26883197. PMCID: PMC4914325
- Srivastava P, Paluch BE, Matsuzaki J, James SR, Collamat-Lai G, Karbach J, Nemeth MJ, Taverna P, Karpf AR, Griffiths EA. Immunomodulatory action of SGI-110, a hypomethylating agent, in acute myeloid leukemia cells and xenografts. Leuk Res. 2014 Nov;38(11):1332-41. Epub 2014 Sep 10. PMID: 25260825.
- Griffiths EA, Golding MC, Srivastava P, Povinelli BJ, James SR, Ford LA, Wetzler M, Wang ES, Nemeth MJ. Pharmacological targeting of β-catenin in normal karyotype acute myeloid leukemia blasts. Haematologica. 2015 Feb;100(2):e49-52. doi: 10.3324/haematol.2014.113118. Epub 2014 Nov 7. PubMed PMID: 25381132; PubMed Central PMCID: PMC4803144