Overcoming acquired resistance to chemotherapy agents in aggressive B-cell lymphoma
The Hernandez-Ilizaliturri Lab set up and developed the translational research lymphoma program based primarily on the use of targeted treatment of lymphoma. Our laboratory projects can be divided in two groups:
- Projects related to understanding the mechanisms of immuno-chemotherapy resistance in B-cell malignancies; and
- Projects focused on developing novel therapeutic strategies for patients with B-cell lymphomas.
Targeting the ubiquin-proteasome system to restore apoptotic threshold in diffuse large B-cell lymphoma (DLBCL)
Based on pre-clinical studies conducted in our laboratory and funded by the NIH, we are completing phase I/II clinical studies evaluating the combination of carfilzomib, a novel proteasome inhibitor, and rituximab in combination with ifosfamide, carboplatin and etoposide (RICE) in relapsed DLBCL patients.
Of interest, the overall response rate to this novel combination (90%) is higher than historical controls (56%). Currently, we are working on reporting the pharmacokinetic analysis of the samples collected during the clinical study.
- Torka P, et al. Carfilzomib combined with rituximab, ifosfamide, carboplatin, and etoposide for relapsed or refractory DLBCL. Blood Adv. 2023 Apr 11;7(7):1146-1155. doi: 10.1182/bloodadvances.2022008543. PMID: 36375132; PMCID: PMC10111346.
- Clinical Trial NCT01959698. Carfilzomib, Rituximab, Ifosfamide, Carboplatin, and Etoposide in Treating Patients With Relapsed or Refractory Stage I-IV Diffuse Large B-cell Lymphoma. Principal Investigator: Francisco Hernandez-Ilizaliturri, MD.
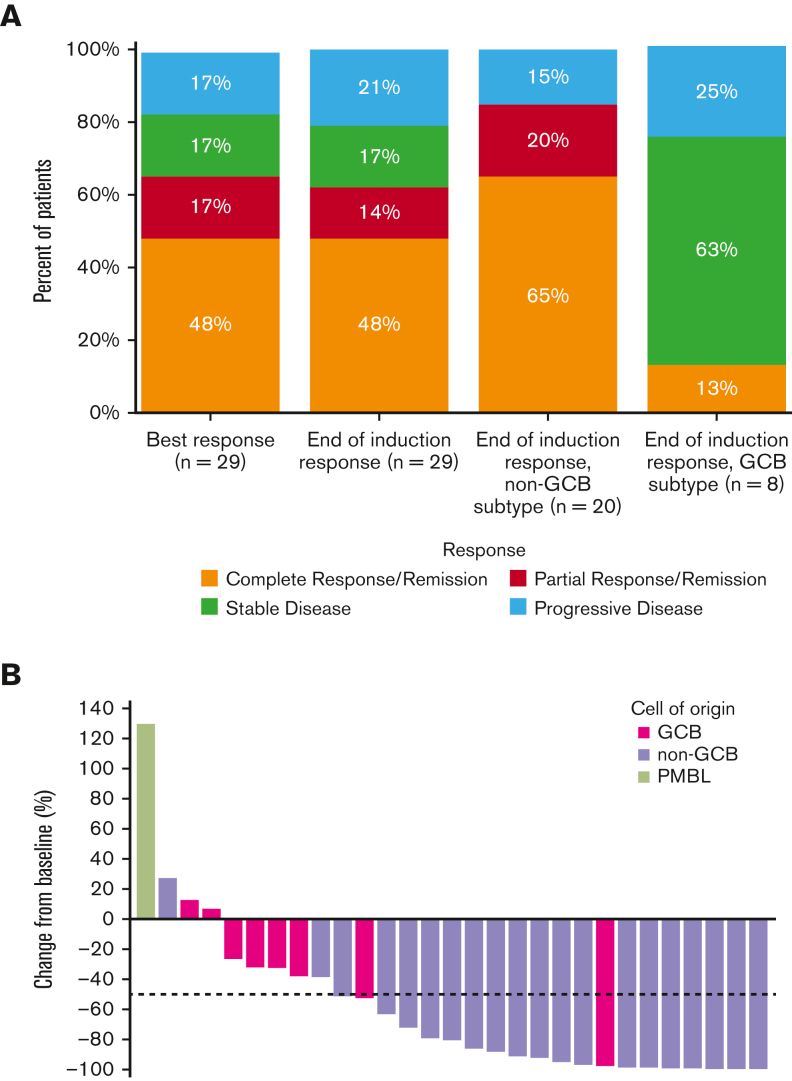
Clinical benefit of lisocabtagene maraleucel (liso-cel), a CD19-directed chimeric antigen receptor (CAR) T cell therapy
We noted on retrospective analysis from our lymphoma database a poor outcome in patients with primary refractory diffuse large B-cell lymphoma, using second line chemotherapy and autologous bone marrow transplant. Because of that, we joined a global clinical trial evaluating the efficacy of CART-19 therapy in patients primary refractory DLBCL.
The randomized clinical trial demonstrated a statistically significant increase in progression free survival (PFS) and overall survival (OS) in favor of lisocabtagene maraleucel when compared to HDC-ASCT. The results of the clinical study have been presented in multiple meetings and were peer-reviewed and published late last year.
- Abramson JS, et al. Lisocabtagene maraleucel as second-line therapy for large B-cell lymphoma: primary analysis of the phase 3 TRANSFORM study. Blood. 2023 Apr 6;141(14):1675-1684.
- Kamdar M, et al. Lisocabtagene maraleucel versus standard of care with salvage chemotherapy followed by autologous stem cell transplantation as second-line treatment in patients with relapsed or refractory large B-cell lymphoma (TRANSFORM): results from an interim analysis of an open-label, randomised, phase 3 trial. Lancet. 2022 Jun 18;399(10343):2294-2308.
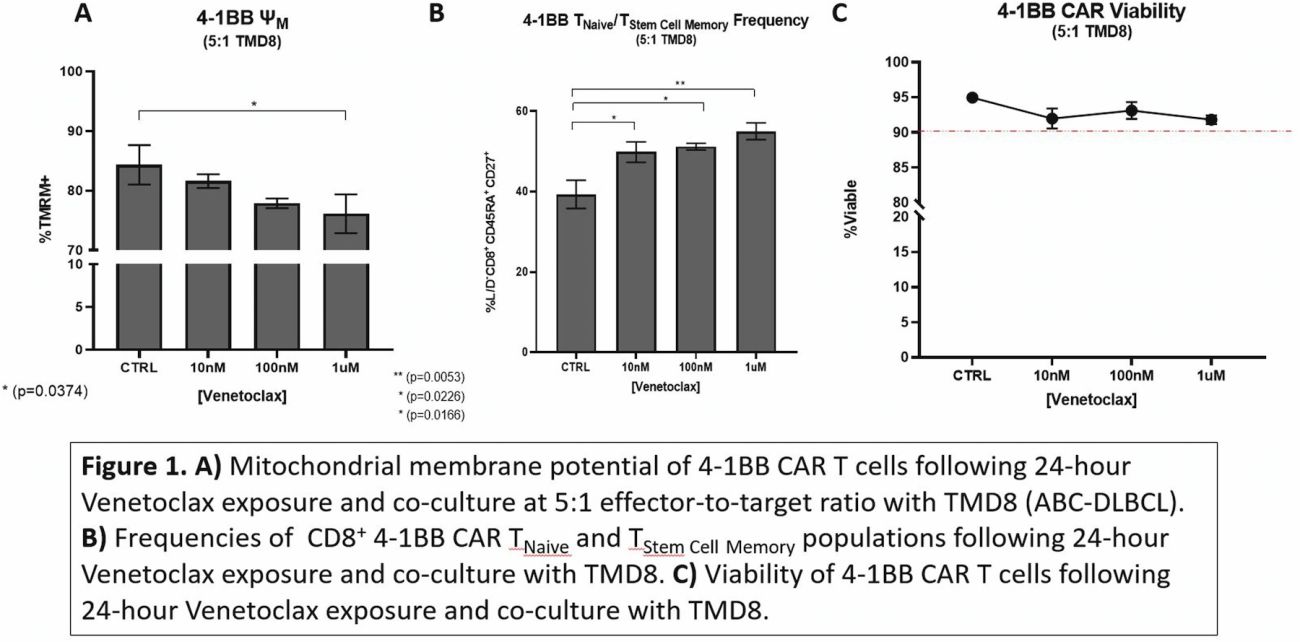
Contribution of Bcl-2 inhibitor Venetoclax toward anti-CD19 CAR-T cell efficacy in relapsed/refractory diffuse large B cell lymphoma
We established a collaboration with the Cleveland Clinic Foundation and Duke University seeking to study biomarkers of response to CART-19 therapy. Using gene sequencing studies, we demonstrated that BCL-2 appears to play a role in poor clinical outcomes in patients treated with CART-19 therapy in the third-line setting.
In order to improve on CART-19 therapy, we are currently evaluating the effects of Bcl-2 inhibition using BH3 mimetics in the anti-tumor activity of CAR-T CD19 cells in lymphoma preclinical models. We demonstrated that the BH3 mimetics enhances the anti-tumor activity of CAR-T CD19 therapy in rituximab-resistant lymphoma pre-clinical models, which may be explained by the effect of the BH3 mimetic’s exposure on CAR-T CD19 cell immunophenotype.
The ability to manipulate Bcl-2 protein expression within anti-CD19 CAR T-cells affords insight into the role that the Bcl-2 family pathway plays within CAR-T CD19 cell biology. In addition to answering fundamental questions of CAR T-cell biology, the combination of Venetoclax and anti-CD19 CAR T-cell therapy may provide a solution to the observed clinical gap, in which anti-CD19 CAR T therapy alone cures less than half of all patients who advance to this second or third line treatment regimen.
- Mandeville T, Mavis C, Gu J, Lemoine N, Bowman K, Olejniczak S, Dey P, Paragh G, Hernandez-Ilizaliturri F. Enhancing a-CD19 CAR T antitumor efficacy using the BH3 mimetic, venetoclax. Blood (2022) 140 (Supplement 1): 7398–7399.
- Mandeville T, Mavis C, Gu J, Olejniczak S, Dey P, Paragh G, Hernandez-Ilizaliturri F. Contribution of Bcl-2 Inhibitor Venetoclax Toward Anti-CD19 CAR T Cell Efficacy in Relapsed/Refractory Diffuse Large B Cell Lymphoma. Blood (2021) 138 (Supplement 1): 1719.
Targeting hexokinase II (HKII)-VDAC complex in rituximab-resistant lymphoma
We discovered that the acquirement of rituximab resistance was associated with a deregulation in the glucose metabolism and an increase in the apoptotic threshold leading to chemotherapy resistance.
Hexokinase (HK) is the first step rate-limiting enzyme of the glycolytic pathway and mediates the phosphorylation of glucose to glucose-6-phosphate (G-6-P), promoting the glycolic pathway. Four isoforms of HK had been characterized. Hexokinase II (HKII), the predominant isoform overexpressed in cancer cells, has a catalytic and a biding domain.
The catalytic domain of HKII promotes glycolysis, while the binding domain protects tumor cells from mitochondrial outer membrane permeability (MOMP) and inhibits mitochondrial-mediated apoptosis. In our current work, we further study the biological and clinical significance of HKII expression in aggressive B-cell lymphoma.
First, we analyzed differences in HKII gene and protein expression in a panel of rituximab-chemotherapy sensitive (RSCL) or resistant (RRCL) diffuse large B-cell lymphoma (DLBCL) and Burkitt’s lymphoma (BL) cell lines. RRCL were found to have higher HKII mRNA and protein levels. Subcellular fractionation of the mitochondria and cytoplasm revealed that most of the HKII was localized in the mitochondria in RRCL.
Moreover, immunoprecipitation studies demonstrated that HKII was bound to the voltage dependent anion channel (VDAC). Of interest, targeting HKII using either 1) a competitive substrate inhibitor, 2) a kinase inhibitor, 3) disruption of its interaction with VDAC, or 4) complete silencing of HKII resulted in a decrease in the decrease in cell viability and re-sensitization of RRCL to chemotherapy agents
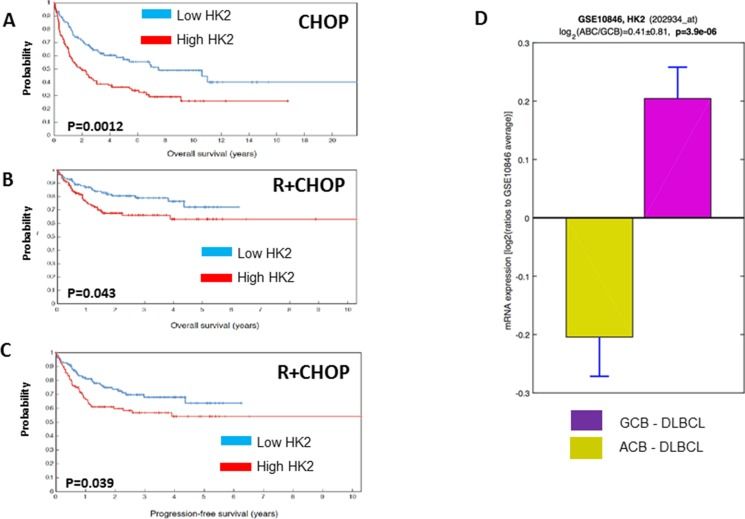
To further assess the contribution of HKII to rituximab/chemotherapy resistance in a more clinically relevant setting, we analyzed gene expression profiling data from 401 patients with DLBCL treated with standard doses of CHOP chemotherapy (N=181) or Rituximab and CHOP (N=220) as front-line therapy. High levels of HKII correlated with a poor prognosis in DLBCL patients. High levels of HKII correlated with a shorter overall survival (OS) and progression free survival (PFS).
Our data suggest that over-expression of HKII contributes to the acquirement of resistance to rituximab and chemotherapy agents in DLBCL B-cell lymphoma. It appears that HKII binding to VDAC and, to a lesser degree, HKII catalytic domain contribute to therapy resistance in lymphoma pre-clinical models. Clinically, higher levels of HKII correlate with a shorter PFS and OS in previously untreated DLBCL in the pre-and post-rituximab era.
Targeting HKII appears to be a challenging but attractive strategy to potentiate the anti-tumor activity of current available monoclonal antibodies or chemotherapy agents in aggressive lymphoma. To this end, we developed several peptides capable of targeting this enzyme and we are conducting pre-clinical studies prior to taking this approach into a phase I clinical trial.
- Gu JJ, et al. Hexokinase II Expression As a Prognosis Marker in Diffuse Large B-Cell Lymphoma (DLBCL) Pre- and Post- the Incorporation of Rituximab to Standard CHOP Chemotherapy. Blood (2019) 134 (Supplement_1): 5233.
- Gu JJ, et al. Up-regulation of hexokinase II contributes to rituximab-chemotherapy resistance and is a clinically relevant target for therapeutic development. Oncotarget. 2017 Dec 19;9(3):4020-4033.
Connect with the Hernandez-Ilizaliturri Lab
Department of Immunology
Roswell Park Comprehensive Cancer Center
Elm and Carlton Streets
Buffalo, NY 14263