AML: An acute need for better-targeted treatments
Acute Myeloid Leukemia (AML) is a hematological malignancy consisting of an abundance of abnormal or poorly differentiated myeloid cells. Standard induction chemotherapy is very successful at eliminating a majority of the disease.
However, in the absence of consolidation therapy, almost all patients will experience a relapse. Also, some patients are too old or weak to receive the full standard induction therapy. AML is a highly heterogeneous disease, posing an obstacle of treatment due to the pairwise cooperativeness among the various mutations.
The fact that standard induction chemotherapy has not greatly changed in the past four decades, along with the heterogeneity of mutations in AML, highlights a vital need for novel treatments that are targeted and better tolerated.
Target: The PARP protein
The PARP protein is involved in the detection and repair of DNA damage. Inhibiting PARP prevents AML cells from repairing damaged DNA and thus induces cell death.
Our lab looks at the potential of the addition of PARP inhibitors to clinically used DNA damaging agents in the hopes of having enhanced anti-leukemic effects on human AML cell lines and primary patient samples.
Our experiments look to find novel, synergistic, and clinically relevant combination treatments with PARP inhibitors that will increase growth inhibition, apoptosis, DNA damage, and cell cycle arrest in vitro and decrease leukemic burden and increase overall survival in vivo.
2021 ASH poster presentation
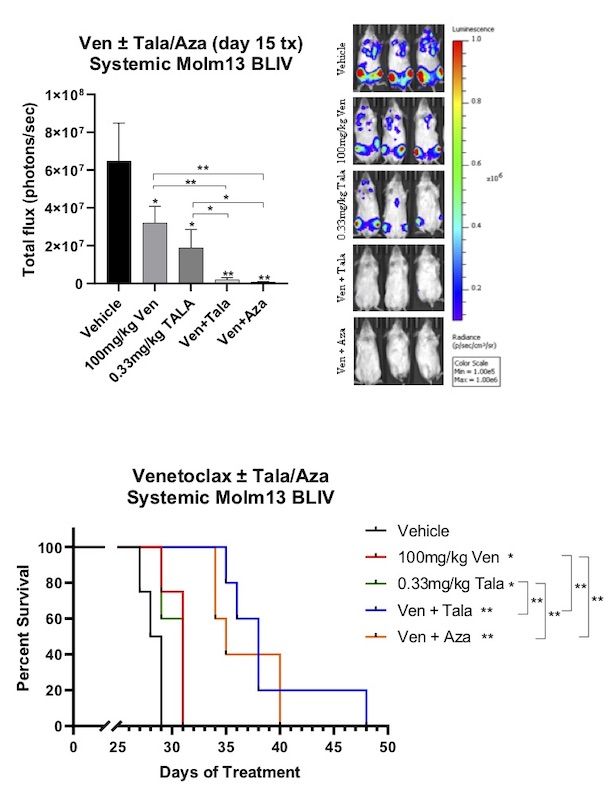
PARP Inhibition with Talazoparib Enhances DNA Damage and Anti-Leukemic Activity of Venetoclax in Preclinical Human Acute Myeloid Leukemia (AML) Models.
- Event: American Society of Hematology Annual Meeting, December 2021
- Authors: Giorgi, M, Portwood, S, Boncek, M, and Wang, ES
Target: Cancer stem cells
The cancer stem cell (CSC) model proposes that populations of rare malignant cells residing within the bone marrow are responsible for maintaining tumors for some cancers such as AML. Secondary engraftment of these CSC populations will re-establish disease in immunocompromised animals.
Our lab has established, evaluated, and now maintains primary AML PDX mice to serve as in vivo models which aim to reflect disease heterogeneity as observed in patients, allowing our research to be clinically relevant.
Connect with the Wang Lab
Department of Immunology
Roswell Park Comprehensive Cancer Center
Elm and Carlton Streets
Buffalo, NY 14263