Cancer of the urethra is rare and occurs when malignant (cancerous) cells grow in the lining of the tubes that connect your kidneys to your bladder. When these mutated cells multiply rapidly it can result in a tumor that blocks the ureter and may spread, or metastasize, to other areas of the body. The first sites of metastasis are usually your external groin or pelvic lymph nodes.
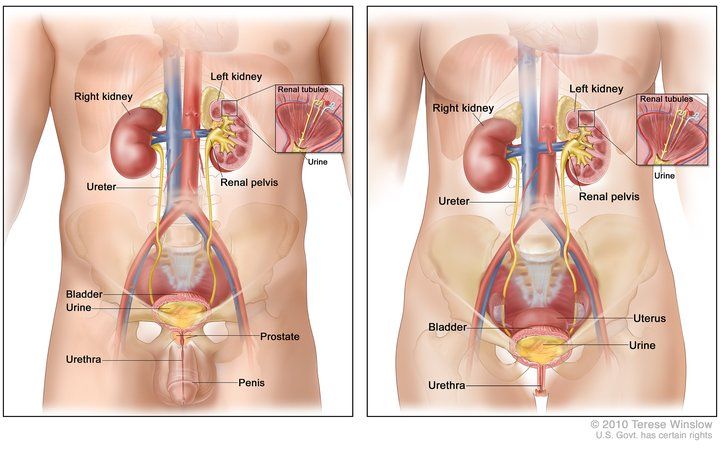
In males and people assigned male at birth (AMAB), the urethra is about eight inches long, passes through your prostate and carries semen and sperm out of your body. In women and individuals assigned female at birth (AFAB), the urethra is about two inches long and located in the vulva, within your labia.
Urethral cancer is most often diagnosed in adults over 60 years old, and in people previously treated for, or with a family history of bladder cancer. Other risk factors include being male or AMAB, or African American.
What causes urethral cancer?
Researchers and doctors don’t know the exact cause of any cancer, but think the following factors may play a role in the development of urethral cancer:
- Chronic inflammation of your urethra, such as frequent urinary tract infections (UTIs)
- Long-term catheterization
- Sexually transmitted diseases (STIs)
- Human papillomavirus (HPV)
- Smoking
Types of urethral cancer
Several kinds of cancer begin in the lining of your urethra, and all fall under the umbrella of urethral cancer:
- Transitional cell carcinoma is the most common type and usually forms in the part of the urethra that is closer to the bladder. It is the same kind of cancer as bladder cancer.
- Squamous cell carcinoma develops at the end of the urethra, near the tip of the penis in males and AMAB people, and near the vagina in women and AFAB people.
- Adenocarcinoma forms in the glands near the urethra and is the most common type of cancer found in the pockets, or outpouchings, of your urethra.
Symptoms of urethral cancer
There are usually no signs of urethral cancer in the earliest stages. In women and AFAB individuals, symptoms may first appear as
- Blood in your urine (hematuria)
- Urinary retention, or difficulty peeing
- Urinary frequency
In males and AMAB individuals, symptoms can include
- Stop-and-go pee stream
- A lump or thickness in your penis, or the space between your genitals and rectum (perineum)
- Clear, white, or off-white urethral discharge
- Enlarged lymph nodes in your groin
Back pain, unexplained weight loss, fatigue and pain when urinating can also be symptoms of urethral cancer.
How is urethral cancer diagnosed?
If you have symptoms of urethral cancer, your healthcare provider will perform a complete physical examination and ask you if you have a personal or family history of bladder or urethral cancer. They may also take blood and urine samples for testing.
Additional tests for diagnosing urethral cancer include:
- Pelvic examination to determine if there are any tumors or abnormalities in your uterus, cervix, vagina, fallopian tubes, ovaries, bladder and rectum.
- Digital rectum examination of your rectum, anus and prostate if you have one.
- Urine cytology to examine your pee for cancer cells from the cells that line your bladder and the part of your urethra that is closest to your bladder.
- Cystoscopy/ureteroscopy to look inside your urethra, ureters, bladder and kidneys using a thin, lighted tube with a lens. Your healthcare provider may also take a tissue sample to examine under a microscope.
- Computed tomography (CT) scan to take detailed pictures of your pelvis and abdomen to create a 3D image of the tissues in this area.
How is urethral cancer treated?
Diagnosis and treatment for urethral cancer should be coordinated by urologists and other physicians who specialize in cancers that affect the urinary system. Therapy depends on the area of your urethra that is affected, if the cancer has spread to other parts of your body and where; your biological sex, general health and whether you have had urethral or bladder cancer before.
Treatment should be individualized and may include one or a combination of the following:
- Surgery to remove the tumor or tumors and, depending on the extent of the cancer, your bladder, urethra, prostate, lymph nodes, vagina or penis may also be removed.
- Radiation therapy to kill cancer cells and keep them from multiplying.
- Chemotherapy that uses cancer-fighting drugs to kill cancer cells and keep them from multiplying.