Myokines mediate androgen deprivation therapy-induced obese frailty
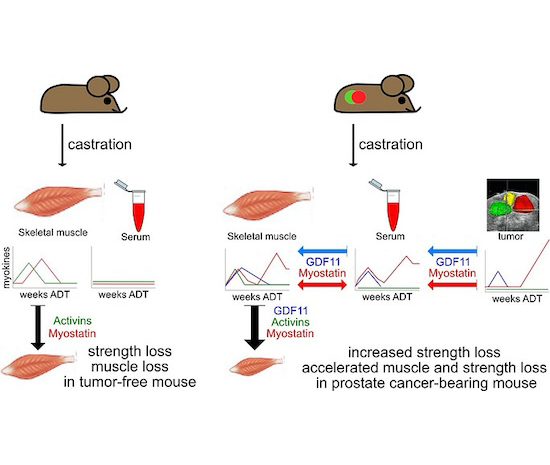
The Nastiuk Lab developed the first mouse model that recapitulates the clinical syndrome of ADT-induced “obese frailty,” including the phenotypic muscle loss and fat gain, as well as the functional loss of strength.
Exploiting this model, we found that muscle regulating TGFß-superfamily cytokines (the myokines) are ADT-regulated. Functionally, pan-myokine blockade abrogates the ADT-induced muscle loss and the functional deficits, indicating that one or more of these myokines may be targets for therapeutic intervention.
While as yet there is no drug intervention to overcome this “obese frailty syndrome” side effect brought on by low testosterone in patients, we are testing whether a combination of diet and exercise can minimize the ADT side effects.
To advance myokine blockade as a drug therapy for patients, we have examined which of these myokine family members is a good therapeutic target to ameliorate ADT-induced sarcopenia in mouse models of prostate cancer.
Surprisingly, we discovered that prostate tumors produce extra myokines, and feedback between tumor myokines and muscle tissue — a dynamic known as an endocrine loop — accelerates the muscle loss. We found that blocking a particular myokine, growth differentiation factor 11, in combination with ADT may be sufficient to prevent strength loss but still control prostate cancer.
We have clinical trials underway to pursue the implications of this work, and these might lead to new therapies to reduce these side effects of prostate cancer treatment.
See the science
- Pan C, Singh S, Sahasrabudhe DM, Chakkalakal JV, Krolewski JJ, Nastiuk KL. TGFβ Superfamily Members Mediate Androgen Deprivation Therapy-Induced Obese Frailty in Male Mice. Endocrinology. 2016 Nov;157(11):4461-4472. doi: 10.1210/en.2016-1580.
- Klose A, Liu W, Paris ND, Forman S, Krolewski JJ, Nastiuk KL, Chakkalakal JV. Castration induces satellite cell activation that contributes to skeletal muscle maintenance. JCSM Rapid Commun. 2018;1(1):e00040.
- Pan C, Jaiswal Agrawal N, Zulia Y, Singh S, Sha K, Mohler JL, Eng KH, Chakkalakal JV, Krolewski JJ, Nastiuk KL. Prostate tumor-derived GDF11 accelerates androgen deprivation therapy-induced sarcopenia. JCI Insight. 2020 Mar 26;5(6):e127018. doi: 10.1172/jci.insight.127018.
Connect with the Nastiuk Lab
Department of Cancer Genetics & Genomics
Roswell Park Comprehensive Cancer Center
Elm and Carlton Streets
Buffalo, NY 14263