Liposomes have been extensively used in the past decade as drug carriers. Desired properties of efficient carriers include the ability to evade the mononuclear phagocyte system (MPS) to prolong the circulation half-life (t1/2), and preferential release of the encapsulated drug at the targeted site. Use of sterically stabilized liposomes has increased the liposome circulation time considerably. “Stealth” liposomes or “cryptosomes” have been used to improve the efficiency of drug delivery by liposomes. Conventional liposomes are intercepted at an early stage of circulation by the mononuclear phagocyte system (MPS). Unlike conventional liposomes, “stealth” or sterically stabilized liposomes show reduced uptake by the MPS, thus prolonging their circulation half-life considerably.
Stealthing of conventional liposomes is done primarily by using polyethylene glycol (PEG) conjugated lipids at different concentrations and compositions. The use of PEG conjugated lipids to form stealth liposomes is a well-accepted process. The predecessors of PEG-lipids, namely GM1 ganglioside and phospatidylinositol, though effective in increasing t1/2 of the conventional liposomes, did not match the superior shielding ability of PEG-lipids. Previous studies have shown that about 1 mole % of PEG lipids of adequate polymer size can completely cover a lipid bilayer surface to provide total inhibition of liposome-cell adhesion. This percentage of PEG-lipids is generally much lower than that commonly used to make stealth liposomes (5–15 mole %) in in vivo applications. However, uncovering or de-stealthing of the PEG coated liposomes at the desired sites has proven to be difficult to achieve.
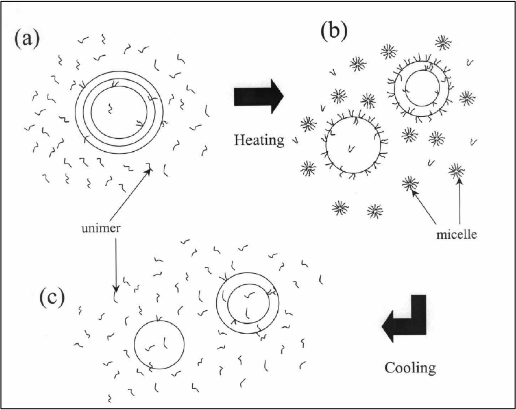
In recent years poloxamers, a group of tri-block co-polymers, have also been used to sterically stabilize liposomes. Poloxamers (sometimes called Pluronics), are polyethylene oxide (PEO)—polypropylene oxide (PPO)—polyethylene oxide tri-block co-polymers of different molecular weights. The hydrophobic PPO group in the middle links the two hydrophilic PEO groups. The amphiphilic nature of the poloxamers makes them extremely useful in various applications as emulsifiers and stabilizers. In an aqueous environment, poloxamers at a given concentration remain as individual (non-associated) co-polymers (referred to herein as ‘monomers’), at temperatures below their critical micellar temperature (CMT). Above the CMT the molecules become more lipophilic, and form micelles with hydrophobic PPO groups at the core of the micelle. Poloxamers of different molecular weights and with different hydrophil-lipophil balance (HLB) have different CMTs. This monomer-to-micellar transition process is extremely temperature-sensitive. With a small change of temperature, the corresponding critical micellar concentration (CMC) may change by several orders of magnitude.
Although poloxamers have been used for stabilizing liposomes, no method is available on utilizing the thermal properties of poloxamers to manipulate liposome-cell adhesion resulting in enhanced retention of sterically protected liposomes at or near the target site.
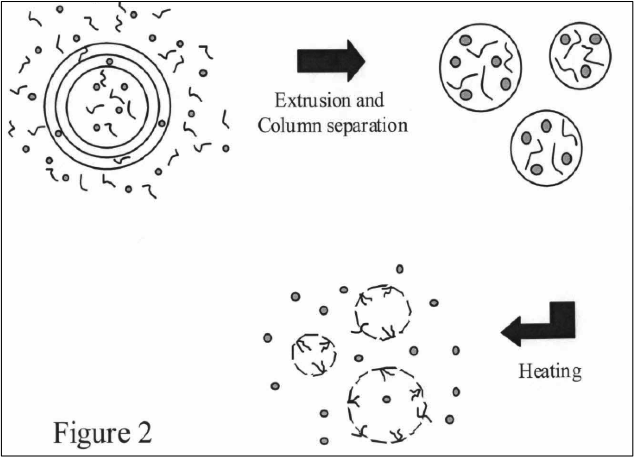
The invention provides a method for targeted delivery of agents comprising the steps of providing a mixture of poloxamer molecules, and liposomes encapsulating the delivery agent; heating the mixture to above the critical micellar temperature (CMT) for the poloxamer, so as to allow a fraction of the poloxamer molecules to form micelles and another fraction of the poloxamer molecules to become incorporated into the liposomes; administering the heated mixture to an individual; and cooling the target site to below the CMT so as to cause the poloxamer molecules forming the micelles and incorporated into the liposomes to dissociate into monomers thereby exposing the liposomal adhesion sites causing the liposomes to be retained at or near the target site.
The invention also provides a liposomal composition for targeted delivery of agents comprising poloxamer molecules and liposome vesicles encapsulating one or more agents for delivery, wherein upon heating of the composition to above the CMT, a fraction of the poloxamer molecules form micelles and another fraction of the poloxamer molecules become incorporated into the liposomes resulting in stealthing of the liposomes such that the liposomal-cell adhesion is reduced, and wherein upon cooling the mixture at the target site to below the CMT of the poloxamer, the poloxamer molecules become dissociated into monomers to expose cell-adhesion sites thereby causing retention of the liposomes at or near the target site.