Pancreatic adenocarcinoma is a highly aggressive cancer with poor prognosis. The majority of patients are diagnosed with advanced-stage disease and have limited treatment options. Thus there is a critical need for more effective systemic chemotherapies.
Over the past few years clinical trials have focused on evaluating various approaches including targered agents and combination therapies. A recently annnounced clinical trial showed improved efficacy of a novel combination of cytotoxic agents. The Metastatic Pancreatic Adenocarcinoma Clinical Trial, or MPCAT, entailed evaluation of Nab-paclitaxel in combination with gemcitabine versus single agent gemcitabine in patients with metastatic pancreatic cancer. Based on promising preclinical data as well as a positive phase I/II study utilizing the same regimen, the trial was a randomized phase III trial conducted across multiple centers globally. A total of 861 patients with no prior therapies for metastatic disease were randomized to receive either nab-paclitaxel (125 mg/m2) and gemcitabine (1000 mg/m2) on days 1,8,15 of a 28-day cycle or single agent gemcitabine (1000 mg/m2 weekly for 7 weeks followed by days 1,8,15 of a 28-day cycle). The primary end point was Overall Survival (OS) and secondary end points included Progression Free Survival (PFS), Overall Response Rate (ORR) as well as evaluation of safety and tolerability.
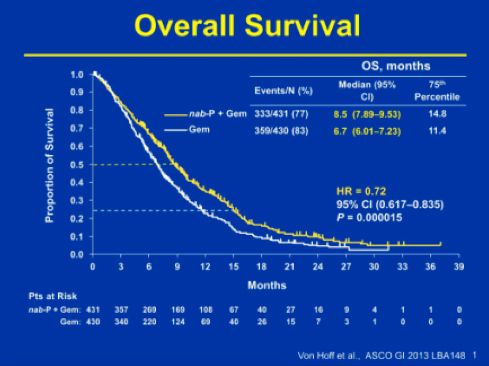
The trial results, as presented at the Amercian Society of Clinical Oncology (ASCO) GI Cancer Symposium in January 2013 showed significant benefit of the combination across all end-points. Median OS was reported to be 8.5 months in the combination arm vs. 6.7 months in the gemcitabine arm (HR 0.72; p = 0.000015). Survival in the combination arm vs. gemcitabine arm was 35% vs. 22% (p = 0.0002) at 12 months and 9% vs. 4% (p = 0.02123) at 24 months. Additionally median PFS and ORR also improved with the addition of nab-paclitaxel (median PFS 5.5 vs. 3.7 months; HR: 0.69; p = 0.000024, ORR 23% vs. 7%; p = 1.1 x 10-10).
Of note, the combination proved to be more effective in the setting of poor prognostic markers as defined by performance status, presence of >3 metastatic sites, liver metastases and Ca 19-9 of > 59x upper limit of normal. The regimen was well tolerated though some grade 3-5 events including neutropenia, fatigue, peripheral neuropathy, and diarrhea occurred more commonly in the combination arm.
This trial established the combination of gemcitabine and abraxane as an effective first-line treatment option for patients with advanced pancreatic cancer. Roswell Park investigators participated and actively enrolled patients in this trial. They continue to investigate novel therapies and targeted therapies with a robust clinical trials portfolio.